Interdomain interactions control Ca2+-dependent potentiation in the cation channel TRPV4
- PMID: 20485495
- PMCID: PMC2867956
- DOI: 10.1371/journal.pone.0010580
Interdomain interactions control Ca2+-dependent potentiation in the cation channel TRPV4
Abstract
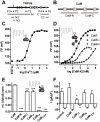
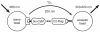
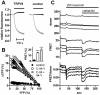
Several Ca(2+)-permeable channels, including the non-selective cation channel TRPV4, are subject to Ca(2+)-dependent facilitation. Although it has been clearly demonstrated in functional experiments that calmodulin (CaM) binding to intracellular domains of TRP channels is involved in this process, the molecular mechanism remains elusive. In this study, we provide experimental evidence for a comprehensive molecular model that explains Ca(2+)-dependent facilitation of TRPV4. In the resting state, an intracellular domain from the channel N terminus forms an autoinhibitory complex with a C-terminal domain that includes a high-affinity CaM binding site. CaM binding, secondary to rises in intracellular Ca(2+), displaces the N-terminal domain which may then form a homologous interaction with an identical domain from a second subunit. This represents a novel potentiation mechanism that may also be relevant in other Ca(2+)-permeable channels.
Conflict of interest statement
Figures





Similar articles
-
A channelopathy mechanism revealed by direct calmodulin activation of TrpV4.Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):9400-5. doi: 10.1073/pnas.1510602112. Epub 2015 Jul 13. Proc Natl Acad Sci U S A. 2015. PMID: 26170305 Free PMC article.
-
Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site.J Biol Chem. 2003 Jul 18;278(29):26541-9. doi: 10.1074/jbc.M302590200. Epub 2003 Apr 30. J Biol Chem. 2003. PMID: 12724311
-
Distinct properties of Ca2+-calmodulin binding to N- and C-terminal regulatory regions of the TRPV1 channel.J Gen Physiol. 2012 Nov;140(5):541-55. doi: 10.1085/jgp.201210810. J Gen Physiol. 2012. PMID: 23109716 Free PMC article.
-
The negative feedback regulation of TRPV4 Ca2+ ion channel function by its C-terminal cytoplasmic domain.Cell Signal. 2012 Oct;24(10):1918-22. doi: 10.1016/j.cellsig.2012.06.008. Epub 2012 Jun 23. Cell Signal. 2012. PMID: 22735813 Review.
-
Structure and function of the calcium-selective TRP channel TRPV6.J Physiol. 2021 May;599(10):2673-2697. doi: 10.1113/JP279024. Epub 2020 Mar 13. J Physiol. 2021. PMID: 32073143 Free PMC article. Review.
Cited by
-
The Calcium Signaling Mechanisms in Arterial Smooth Muscle and Endothelial Cells.Compr Physiol. 2021 Apr 1;11(2):1831-1869. doi: 10.1002/cphy.c200030. Compr Physiol. 2021. PMID: 33792900 Free PMC article.
-
Increased basal activity is a key determinant in the severity of human skeletal dysplasia caused by TRPV4 mutations.PLoS One. 2011 May 5;6(5):e19533. doi: 10.1371/journal.pone.0019533. PLoS One. 2011. PMID: 21573172 Free PMC article.
-
Classical Transient Receptor Potential 1 (TRPC1): Channel or Channel Regulator?Cells. 2014 Sep 29;3(4):939-62. doi: 10.3390/cells3040939. Cells. 2014. PMID: 25268281 Free PMC article. Review.
-
A channelopathy mechanism revealed by direct calmodulin activation of TrpV4.Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):9400-5. doi: 10.1073/pnas.1510602112. Epub 2015 Jul 13. Proc Natl Acad Sci U S A. 2015. PMID: 26170305 Free PMC article.
-
Transient receptor potential vanilloid 4 (TRPV4) activation by arachidonic acid requires protein kinase A-mediated phosphorylation.J Biol Chem. 2018 Apr 6;293(14):5307-5322. doi: 10.1074/jbc.M117.811075. Epub 2018 Feb 8. J Biol Chem. 2018. PMID: 29462784 Free PMC article.
References
-
- Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca(2+)-dependent feedback inhibition of store-operated Ca(2+) influx by interaction with a site in the C terminus of TrpC1. Mol Cell. 2002;9:739–750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

