Antiretroviral treatment start-time during primary SIV(mac) infection in macaques exerts a different impact on early viral replication and dissemination
- PMID: 20485497
- PMCID: PMC2868019
- DOI: 10.1371/journal.pone.0010570
Antiretroviral treatment start-time during primary SIV(mac) infection in macaques exerts a different impact on early viral replication and dissemination
Abstract
Background: The time of infection is rarely known in human cases; thus, the effects of delaying the initiation of antiretroviral therapy (ART) on the peripheral viral load and the establishment of viral reservoirs are poorly understood.
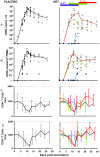
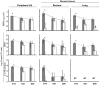
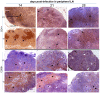
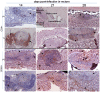
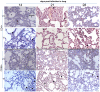
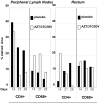
Methodology/principal findings: Six groups of macaques, infected intravenously with SIV(mac251), were given placebo or antiretroviral therapy to explore reservoir establishment; macaques were treated for 2 weeks, with treatment starting 4 hours, 7 or 14 days after infection. Viral replication and dissemination were measured in the gut (rectum), in the lung and in blood and lymphoid tissues (peripheral lymph nodes), by quantifying viral RNA, DNA and 2LTR circles. We used immunohistochemistry (CD4 and CD68) to assess the impact of these treatments on the relative amount of virus target cells in tissue. Treatment that was started 4 hours post-infection (pi) decreased viral replication and dissemination in blood and tissue samples, which were assessed on day 14 (RNA/DNA/2LTR circles). The virus remained detectable and lymphoid tissues were activated in LN and the gut in both placebo- and ART-treated animals. Viral RNA in plasma continued to be lower in macaques treated seven days after infection; however, this was not the case for viral DNA in peripheral blood mononuclear cells. There was a small but significant difference in RNA and DNA levels in tissues between placebo- and ART-treated animals on day 21. When started 14 days after infection, treatment resulted in a limited decrease in the plasma viral load.
Conclusions: Treatment that was started 4 hours after infection significantly reduced viral replication and dissemination. When started 7 days after infection, it was of slight virological benefit in peripheral blood and in tissues, and treatment was even less effective if started 14 days pi. These data favor starting ART no longer than one week after intravenous SIV(mac251) exposure.
Conflict of interest statement
Figures
References
-
- Smith DK, Grohskopf LA, Black RJ, Auerbach JD, Veronese F, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54:1–20. - PubMed
-
- Yeni P. Paris: Medecine-Sciences Flammarion; 2008. Prise en charge medicale des personnes infectees par le VIH.412. Recommandations du groupe d'experts.
-
- Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

