Significant impact of sequence variations in the nucleoprotein on CD8 T cell-mediated cross-protection against influenza A virus infections
- PMID: 20485501
- PMCID: PMC2868023
- DOI: 10.1371/journal.pone.0010583
Significant impact of sequence variations in the nucleoprotein on CD8 T cell-mediated cross-protection against influenza A virus infections
Abstract
Background: Memory CD8 T cells to influenza A viruses are widely detectable in healthy human subjects and broadly cross-reactive for serologically distinct influenza A virus subtypes. However, it is not clear to what extent such pre-existing cellular immunity can provide cross-subtype protection against novel emerging influenza A viruses.
Methodology/principal findings: We show in the mouse model that naturally occurring sequence variations of the conserved nucleoprotein of the virus significantly impact cross-protection against lethal disease in vivo. When priming and challenge viruses shared identical sequences of the immunodominant, protective NP(366)/D(b) epitope, strong cross-subtype protection was observed. However, when they did not share complete sequence identity in this epitope, cross-protection was considerably reduced. Contributions of virus-specific antibodies appeared to be minimal under these circumstances. Detailed analysis revealed that the magnitude of the memory CD8 T cell response triggered by the NP(366)/D(b) variants was significantly lower than those triggered by the homologous NP(366)/D(b) ligand. It appears that strict specificity of a dominant public TCR to the original NP(366)/D(b) ligand may limit the expansion of cross-reactive memory CD8 T cells to the NP(366)/D(b) variants.
Conclusions/significance: Pre-existing CD8 T cell immunity may provide substantial cross-protection against heterosubtypic influenza A viruses, provided that the priming and the subsequent challenge viruses share the identical sequences of the immunodominant, protective CTL epitopes.
Conflict of interest statement
Figures
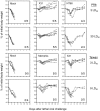

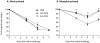
Similar articles
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Diversifying T-cell responses: safeguarding against pandemic influenza with mosaic nucleoprotein.J Virol. 2025 Mar 18;99(3):e0086724. doi: 10.1128/jvi.00867-24. Epub 2025 Feb 3. J Virol. 2025. PMID: 39898643 Free PMC article.
-
Induction of heterosubtypic cross-protection against influenza by a whole inactivated virus vaccine: the role of viral membrane fusion activity.PLoS One. 2012;7(1):e30898. doi: 10.1371/journal.pone.0030898. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303469 Free PMC article.
-
Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines.J Biomed Biotechnol. 2011;2011:939860. doi: 10.1155/2011/939860. Epub 2011 Oct 5. J Biomed Biotechnol. 2011. PMID: 22007149 Free PMC article. Review.
-
Virus-specific T cells as correlate of (cross-)protective immunity against influenza.Vaccine. 2015 Jan 15;33(4):500-6. doi: 10.1016/j.vaccine.2014.11.054. Epub 2014 Dec 9. Vaccine. 2015. PMID: 25498210 Review.
Cited by
-
Induction of cross-protection against influenza A virus by DNA prime-intranasal protein boost strategy based on nucleoprotein.Virol J. 2012 Nov 23;9:286. doi: 10.1186/1743-422X-9-286. Virol J. 2012. PMID: 23173785 Free PMC article.
-
Mutations Conferring Increased Sensitivity to Tripartite Motif 22 Restriction Accumulated Progressively in the Nucleoprotein of Seasonal Influenza A (H1N1) Viruses between 1918 and 2009.mSphere. 2018 Apr 4;3(2):e00110-18. doi: 10.1128/mSphere.00110-18. Print 2018 Apr 25. mSphere. 2018. PMID: 29624498 Free PMC article.
-
Subsisting H1N1 influenza memory responses are insufficient to protect from pandemic H1N1 influenza challenge in C57BL/6 mice.J Gen Virol. 2013 Aug;94(Pt 8):1701-1711. doi: 10.1099/vir.0.049494-0. Epub 2013 Apr 11. J Gen Virol. 2013. PMID: 23580424 Free PMC article.
-
Safety, Immunogenicity, and Protective Efficacy of a Chimeric A/B Live Attenuated Influenza Vaccine in a Mouse Model.Microorganisms. 2021 Jan 27;9(2):259. doi: 10.3390/microorganisms9020259. Microorganisms. 2021. PMID: 33513862 Free PMC article.
-
Engineering temperature sensitive live attenuated influenza vaccines from emerging viruses.Vaccine. 2012 May 21;30(24):3691-702. doi: 10.1016/j.vaccine.2012.03.025. Epub 2012 Mar 24. Vaccine. 2012. PMID: 22449422 Free PMC article.
References
-
- Epstein SL. Control of influenza virus infection by immunity to conserved viral features. Expert Rev Anti Infect Ther. 2003;1:627–638. - PubMed
-
- Yoshikawa T, Matsuo K, Matsuo K, Suzuki Y, Nomoto A, et al. Total viral genome copies and virus-Ig complexes after infection with influenza virus in the nasal secretions of immunized mice. J Gen Virol. 2004;85:2339–2346. - PubMed
-
- Roose K, Fiers W, Saelens X. Pandemic preparedness: toward a universal influenza vaccine. Drug News Perspect. 2009;22:80–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

