The readability of information and consent forms in clinical research in France
- PMID: 20485505
- PMCID: PMC2868027
- DOI: 10.1371/journal.pone.0010576
The readability of information and consent forms in clinical research in France
Abstract
Background: Quantitative tools have been developed to evaluate the readability of written documents and have been used in several studies to evaluate information and consent forms. These studies all showed that such documents had a low level of readability. Our objective is to evaluate the readability of Information and Consent Forms (ICFs) used in clinical research.
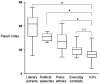
Methods and findings: Clinical research protocols were collected from four public clinical research centers in France. Readability was evaluated based on three criteria: the presence of an illustration, the length of the text and its Flesch score. Potential effects of protocol characteristics on the length and readability of the ICFs were determined. Medical and statutory parts of the ICF form were analyzed separately. The readability of these documents was compared with that of everyday contracts, press articles, literary extracts and political speeches. We included 209 protocols and the corresponding 275 ICFs. The median length was 1304 words. Their Flesch readability scores were low (median: 24), and only about half that of selected press articles. ICF s for industrially sponsored and randomized protocols were the longest and had the highest readability scores. More than half (52%) of the text in ICFs concerned medical information, and this information was statistically (p<0.05) more readable (Flesch: 28) than statutory information (Flesch: 21).
Conclusion: Regardless of the field of research, the ICFs for protocols included had poor readability scores. However, a prospective analysis of this test in French should be carried out before it is put into general use.
Conflict of interest statement
Figures

References
-
- International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. 1996. Guideline for Good Clinical Practice. E6 R1. June 1996. Available: http://www.ich.org/ - PMC - PubMed
-
- World Medical Association. 2008. “Declaration of Helsinki: ethical principles for research involving human subjects”, October 2008. Available: http://www.wma.net/
-
- World Health Organization and CIOMS. 1981. The WHO/CIOMS guidelines, proposed international guidelines for biomedical research involving human subjects, 1981. Availabe: http://www.tbethics.org/
-
- Additional protocol to the convention on human rights and biomedicine, concerning biomedical research, January 2005. Available: http://conventions.coe.int/
-
- Bailin A, Grafstein A. The linguistic assumptions underlying readability formulae: a critique. Language and communication. 2001;21:285–301.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

