A male with unilateral microphthalmia reveals a role for TMX3 in eye development
- PMID: 20485507
- PMCID: PMC2868029
- DOI: 10.1371/journal.pone.0010565
A male with unilateral microphthalmia reveals a role for TMX3 in eye development
Abstract
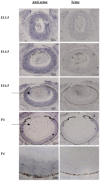
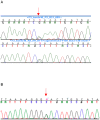
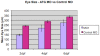
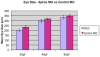
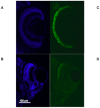
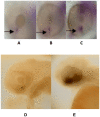
Anophthalmia and microphthalmia are important birth defects, but their pathogenesis remains incompletely understood. We studied a patient with severe unilateral microphthalmia who had a 2.7 Mb deletion at chromosome 18q22.1 that was inherited from his mother. In-situ hybridization showed that one of the deleted genes, TMX3, was expressed in the retinal neuroepithelium and lens epithelium in the developing murine eye. We re-sequenced TMX3 in 162 patients with anophthalmia or microphthalmia, and found two missense substitutions in unrelated patients: c.116G>A, predicting p.Arg39Gln, in a male with unilateral microphthalmia and retinal coloboma, and c.322G>A, predicting p.Asp108Asn, in a female with unilateral microphthalmia and severe micrognathia. We used two antisense morpholinos targeted against the zebrafish TMX3 orthologue, zgc:110025, to examine the effects of reduced gene expression in eye development. We noted that the morphant larvae resulting from both morpholinos had significantly smaller eye sizes and reduced labeling with islet-1 antibody directed against retinal ganglion cells at 2 days post fertilization. Co-injection of human wild type TMX3 mRNA rescued the small eye phenotype obtained with both morpholinos, whereas co-injection of human TMX3(p.Arg39Gln) mutant mRNA, analogous to the mutation in the patient with microphthalmia and coloboma, did not rescue the small eye phenotype. Our results show that haploinsufficiency for TMX3 results in a small eye phenotype and represents a novel genetic cause of microphthalmia and coloboma. Future experiments to determine if other thioredoxins are important in eye morphogenesis and to clarify the mechanism of function of TMX3 in eye development are warranted.
Conflict of interest statement
Figures
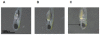


References
-
- Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. - PubMed
-
- Rainger J, Van Heyningen V, FitzPatrick DR. Development of the Eye. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn Errors of Development. Oxford: Oxford University Press; 2008. pp. 94–106.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

