Listeria monocytogenes internalin B activates junctional endocytosis to accelerate intestinal invasion
- PMID: 20485518
- PMCID: PMC2869327
- DOI: 10.1371/journal.ppat.1000900
Listeria monocytogenes internalin B activates junctional endocytosis to accelerate intestinal invasion
Abstract
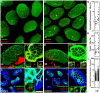
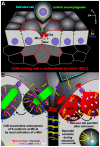
Listeria monocytogenes (Lm) uses InlA to invade the tips of the intestinal villi, a location at which cell extrusion generates a transient defect in epithelial polarity that exposes the receptor for InlA, E-cadherin, on the cell surface. As the dying cell is removed from the epithelium, the surrounding cells reorganize to form a multicellular junction (MCJ) that Lm exploits to find its basolateral receptor and invade. By examining individual infected villi using 3D-confocal imaging, we uncovered a novel role for the second major invasin, InlB, during invasion of the intestine. We infected mice intragastrically with isogenic strains of Lm that express or lack InlB and that have a modified InlA capable of binding murine E-cadherin and found that Lm lacking InlB invade the same number of villi but have decreased numbers of bacteria within each infected villus tip. We studied the mechanism of InlB action at the MCJs of polarized MDCK monolayers and find that InlB does not act as an adhesin, but instead accelerates bacterial internalization after attachment. InlB locally activates its receptor, c-Met, and increases endocytosis of junctional components, including E-cadherin. We show that MCJs are naturally more endocytic than other sites of the apical membrane, that endocytosis and Lm invasion of MCJs depends on functional dynamin, and that c-Met activation by soluble InlB or hepatocyte growth factor (HGF) increases MCJ endocytosis. Also, in vivo, InlB applied through the intestinal lumen increases endocytosis at the villus tips. Our findings demonstrate a two-step mechanism of synergy between Lm's invasins: InlA provides the specificity of Lm adhesion to MCJs at the villus tips and InlB locally activates c-Met to accelerate junctional endocytosis and bacterial invasion of the intestine.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, et al. Entry of Listeria monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. - PubMed
-
- Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. - PubMed
-
- Temm-Grove CJ, Jockusch BM, Rohde M, Niebuhr K, Chakraborty T, et al. Exploitation of microfilament proteins by Listeria monocytogenes: microvillus-like composition of the comet tails and vectorial spreading in polarized epithelial sheets. J Cell Sci. 1994;107 (Pt 10):2951–2960. - PubMed
-
- Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7:333–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

