Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting
- PMID: 20485519
- PMCID: PMC2869328
- DOI: 10.1371/journal.pgen.1000948
Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting
Abstract
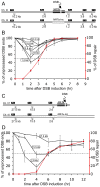
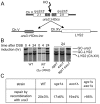
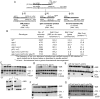
The formation of single-stranded DNA (ssDNA) at double-strand break (DSB) ends is essential in repair by homologous recombination and is mediated by DNA helicases and nucleases. Here we estimated the length of ssDNA generated during DSB repair and analyzed the consequences of elimination of processive resection pathways mediated by Sgs1 helicase and Exo1 nuclease on DSB repair fidelity. In wild-type cells during allelic gene conversion, an average of 2-4 kb of ssDNA accumulates at each side of the break. Longer ssDNA is formed during ectopic recombination or break-induced replication (BIR), reflecting much slower repair kinetics. This relatively extensive resection may help determine sequences involved in homology search and prevent recombination within short DNA repeats next to the break. In sgs1Delta exo1Delta mutants that form only very short ssDNA, allelic gene conversion decreases 5-fold and DSBs are repaired by BIR or de novo telomere formation resulting in loss of heterozygosity. The absence of the telomerase inhibitor, PIF1, increases de novo telomere pathway usage to about 50%. Accumulation of Cdc13, a protein recruiting telomerase, at the break site increases in sgs1Delta exo1Delta, and the requirement of the Ku complex for new telomere formation is partially bypassed. In contrast to this decreased and alternative DSB repair, the efficiency and accuracy of gene targeting increases dramatically in sgs1Delta exo1Delta cells, suggesting that transformed DNA is very stable in these mutants. Altogether these data establish a new role for processive resection in the fidelity of DSB repair.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


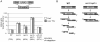
References
-
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

