Differing mechanisms underlie sexual size-dimorphism in two populations of a sex-changing fish
- PMID: 20485547
- PMCID: PMC2868897
- DOI: 10.1371/journal.pone.0010616
Differing mechanisms underlie sexual size-dimorphism in two populations of a sex-changing fish
Abstract
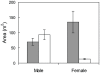
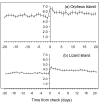
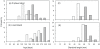
Variability in the density of groups within a patchy environment lead to differences in interaction rates, growth dynamics and social organization. In protogynous hermaphrodites there are hypothesised trade-offs among sex-specific growth, reproductive output and mortality. When differences in density lead to changes to social organization the link between growth and the timing of sex-change is predicted to change. The present study explores this prediction by comparing the social organisation and sex-specific growth of two populations of a protogynous tropical wrasse, Halichoeres miniatus, which differ in density. At a low density population a strict harem structure was found, where males maintained a tight monopoly of access and spawning rights to females. In contrast, at a high density population a loosely organised system prevailed, where females could move throughout multiple male territories. Otolith microstructure revealed the species to be annual and deposit an otolith check associated with sex-change. Growth trajectories suggested that individuals that later became males in both populations underwent a growth acceleration at sex-change. Moreover, in the high density population, individuals that later became males were those individuals that had the largest otolith size at hatching and consistently deposited larger increments throughout early larval, juvenile and female life. This study demonstrates that previous growth history and growth rate changes associated with sex change can be responsible for the sexual dimorphism typically found in sex-changing species, and that the relative importance of these may be socially constrained.
Conflict of interest statement
Figures



References
-
- Charnov EL. Princeton University Press; 1982. The theory of sex allocation. Princeton. - PubMed
-
- Iwasa Y. Sex change evolution and cost of reproduction. Behav Ecol. 1991;2:56–68.
-
- Munday PL, Buston PM, Warner RR. Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol. 2006a;21:89–95. - PubMed
-
- Buston P. Size and growth modification in clownfish. Nature. 2003;424:145–146. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

