A model for genetic and epigenetic regulatory networks identifies rare pathways for transcription factor induced pluripotency
- PMID: 20485562
- PMCID: PMC2869311
- DOI: 10.1371/journal.pcbi.1000785
A model for genetic and epigenetic regulatory networks identifies rare pathways for transcription factor induced pluripotency
Abstract
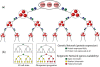
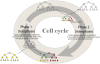
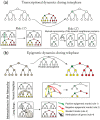
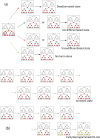
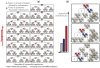
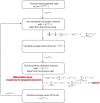
With relatively low efficiency, differentiated cells can be reprogrammed to a pluripotent state by ectopic expression of a few transcription factors. An understanding of the mechanisms that underlie data emerging from such experiments can help design optimal strategies for creating pluripotent cells for patient-specific regenerative medicine. We have developed a computational model for the architecture of the epigenetic and genetic regulatory networks which describes transformations resulting from expression of reprogramming factors. Importantly, our studies identify the rare temporal pathways that result in induced pluripotent cells. Further experimental tests of predictions emerging from our model should lead to fundamental advances in our understanding of how cellular identity is maintained and transformed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
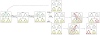
Similar articles
-
Maintaining differentiated cellular identity.Nat Rev Genet. 2012 May 18;13(6):429-39. doi: 10.1038/nrg3209. Nat Rev Genet. 2012. PMID: 22596319 Review.
-
Pluripotency, Differentiation, and Reprogramming: A Gene Expression Dynamics Model with Epigenetic Feedback Regulation.PLoS Comput Biol. 2015 Aug 26;11(8):e1004476. doi: 10.1371/journal.pcbi.1004476. eCollection 2015 Aug. PLoS Comput Biol. 2015. PMID: 26308610 Free PMC article.
-
Molecular basis of pluripotency.Hum Mol Genet. 2008 Apr 15;17(R1):R23-7. doi: 10.1093/hmg/ddn050. Hum Mol Genet. 2008. PMID: 18632692 Review.
-
A common molecular logic determines embryonic stem cell self-renewal and reprogramming.EMBO J. 2019 Jan 3;38(1):e100003. doi: 10.15252/embj.2018100003. Epub 2018 Nov 27. EMBO J. 2019. PMID: 30482756 Free PMC article.
-
Parallel gateways to pluripotency: open chromatin in stem cells and development.Curr Opin Genet Dev. 2010 Oct;20(5):492-9. doi: 10.1016/j.gde.2010.06.002. Epub 2010 Jul 2. Curr Opin Genet Dev. 2010. PMID: 20598875 Free PMC article. Review.
Cited by
-
Probing the role of stochasticity in a model of the embryonic stem cell: heterogeneous gene expression and reprogramming efficiency.BMC Syst Biol. 2012 Aug 13;6:98. doi: 10.1186/1752-0509-6-98. BMC Syst Biol. 2012. PMID: 22889237 Free PMC article.
-
Evolution of hierarchy and irreversibility in theoretical cell differentiation model.PNAS Nexus. 2023 Dec 22;3(1):pgad454. doi: 10.1093/pnasnexus/pgad454. eCollection 2024 Jan. PNAS Nexus. 2023. PMID: 38205032 Free PMC article.
-
Scalable control of developmental timetables by epigenetic switching networks.J R Soc Interface. 2021 Jul;18(180):20210109. doi: 10.1098/rsif.2021.0109. Epub 2021 Jul 21. J R Soc Interface. 2021. PMID: 34283940 Free PMC article.
-
Multi-scale Dynamical Modeling of T Cell Development from an Early Thymic Progenitor State to Lineage Commitment.Cell Rep. 2021 Jan 12;34(2):108622. doi: 10.1016/j.celrep.2020.108622. Cell Rep. 2021. PMID: 33440162 Free PMC article.
-
Epigenetic cell memory: The gene's inner chromatin modification circuit.PLoS Comput Biol. 2022 Apr 6;18(4):e1009961. doi: 10.1371/journal.pcbi.1009961. eCollection 2022 Apr. PLoS Comput Biol. 2022. PMID: 35385468 Free PMC article.
References
-
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. - PubMed
-
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. - PubMed
-
- Park IH, Zhao R, West JA, Yabuuchi A, Huo HG, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–U141. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

