Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans
- PMID: 20485569
- PMCID: PMC2869318
- DOI: 10.1371/journal.pgen.1000953
Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans
Abstract
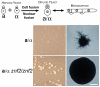
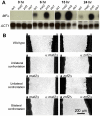
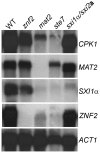
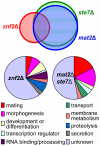
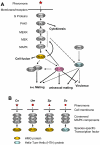
Cryptococcus neoformans is a human fungal pathogen that undergoes a dimorphic transition from a unicellular yeast to multicellular hyphae during opposite sex (mating) and unisexual reproduction (same-sex mating). Opposite- and same-sex mating are induced by similar environmental conditions and involve many shared components, including the conserved pheromone sensing Cpk1 MAPK signal transduction cascade that governs the dimorphic switch in C. neoformans. However, the homeodomain cell identity proteins Sxi1alpha/Sxi2a encoded by the mating type locus that are essential for completion of sexual reproduction following cell-cell fusion during opposite-sex mating are dispensable for same-sex mating. Therefore, identification of downstream targets of the Cpk1 MAPK pathway holds the key to understanding molecular mechanisms governing the two distinct developmental fates. Thus far, homology-based approaches failed to identify downstream transcription factors which may therefore be species-specific. Here, we applied insertional mutagenesis via Agrobacterium-mediated transformation and transcription analysis using whole genome microarrays to identify factors involved in C. neoformans differentiation. Two transcription factors, Mat2 and Znf2, were identified as key regulators of hyphal growth during same- and opposite-sex mating. Mat2 is an HMG domain factor, and Znf2 is a zinc finger protein; neither is encoded by the mating type locus. Genetic, phenotypic, and transcriptional analyses of Mat2 and Znf2 provide evidence that Mat2 is a downstream transcription factor of the Cpk1 MAPK pathway whereas Znf2 functions as a more terminal hyphal morphogenesis determinant. Although the components of the MAPK pathway including Mat2 are not required for virulence in animal models, Znf2, as a hyphal morphology determinant, is a negative regulator of virulence. Further characterization of these elements and their target circuits will reveal genes controlling biological processes central to fungal development and virulence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans.PLoS Genet. 2013;9(8):e1003688. doi: 10.1371/journal.pgen.1003688. Epub 2013 Aug 15. PLoS Genet. 2013. PMID: 23966871 Free PMC article.
-
Pheromone independent unisexual development in Cryptococcus neoformans.PLoS Genet. 2017 May 3;13(5):e1006772. doi: 10.1371/journal.pgen.1006772. eCollection 2017 May. PLoS Genet. 2017. PMID: 28467481 Free PMC article.
-
Unraveling the cryptic functions of mitogen-activated protein kinases Cpk2 and Mpk2 in Cryptococcus neoformans.mBio. 2024 Jul 17;15(7):e0115624. doi: 10.1128/mbio.01156-24. Epub 2024 Jun 14. mBio. 2024. PMID: 38874410 Free PMC article.
-
Unisexual versus bisexual mating in Cryptococcus neoformans: Consequences and biological impacts.Fungal Genet Biol. 2015 May;78:65-75. doi: 10.1016/j.fgb.2014.08.008. Epub 2014 Aug 27. Fungal Genet Biol. 2015. PMID: 25173822 Free PMC article. Review.
-
[Mating types, sexual reproduction and ploidy in fungi: effects on virulence].Mikrobiyol Bul. 2009 Jul;43(3):507-13. Mikrobiyol Bul. 2009. PMID: 19795629 Review. Turkish.
Cited by
-
Sexual Differentiation Is Coordinately Regulated by Cryptococcus neoformans CRK1 and GAT1.Genes (Basel). 2020 Jun 19;11(6):669. doi: 10.3390/genes11060669. Genes (Basel). 2020. PMID: 32575488 Free PMC article.
-
Fungal mating pheromones: choreographing the dating game.Fungal Genet Biol. 2011 Jul;48(7):668-76. doi: 10.1016/j.fgb.2011.04.001. Epub 2011 Apr 8. Fungal Genet Biol. 2011. PMID: 21496492 Free PMC article. Review.
-
A silver bullet in a golden age of functional genomics: the impact of Agrobacterium-mediated transformation of fungi.Fungal Biol Biotechnol. 2017 Sep 26;4:6. doi: 10.1186/s40694-017-0035-0. eCollection 2017. Fungal Biol Biotechnol. 2017. PMID: 28955474 Free PMC article. Review.
-
Analysis of Cryptococcus neoformans sexual development reveals rewiring of the pheromone-response network by a change in transcription factor identity.Genetics. 2012 Jun;191(2):435-49. doi: 10.1534/genetics.112.138958. Epub 2012 Mar 30. Genetics. 2012. PMID: 22466042 Free PMC article.
-
The link between morphotype transition and virulence in Cryptococcus neoformans.PLoS Pathog. 2012;8(6):e1002765. doi: 10.1371/journal.ppat.1002765. Epub 2012 Jun 21. PLoS Pathog. 2012. PMID: 22737071 Free PMC article.
References
-
- Dohlman HG, Slessareva JE. Pheromone signaling pathways in yeast. Sci STKE. 2006;2006:cm6. - PubMed
-
- Schwartz MA, Madhani HD. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu Rev Genet. 2004;38:725–748. - PubMed
-
- Davidson RC, Nichols CB, Cox GM, Perfect JR, Heitman J. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2003;49:469–485. - PubMed
-
- Bardwell L, Cook JG, Inouye CJ, Thorner J. Signal propagation and regulation in the mating pheromone response pathway of the yeast Saccharomyces cerevisiae. Dev Biol. 1994;166:363–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

