A review of new developments in the Friedel-Crafts alkylation - From green chemistry to asymmetric catalysis
- PMID: 20485588
- PMCID: PMC2870981
- DOI: 10.3762/bjoc.6.6
A review of new developments in the Friedel-Crafts alkylation - From green chemistry to asymmetric catalysis
Abstract
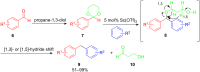
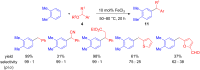
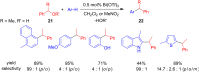
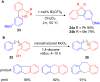
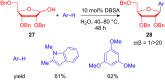
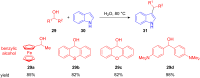
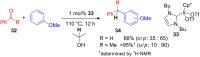
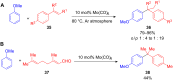
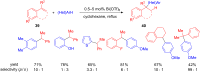
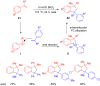
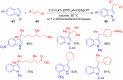
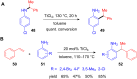
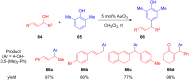
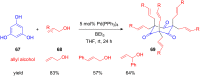
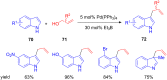
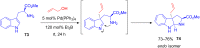
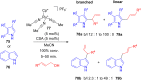
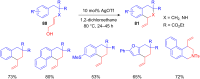
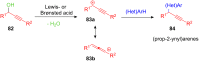
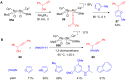
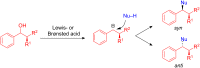
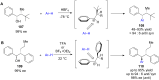
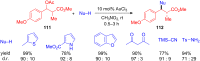
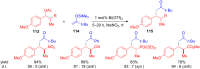
The development of efficient Friedel-Crafts alkylations of arenes and heteroarenes using only catalytic amounts of a Lewis acid has gained much attention over the last decade. The new catalytic approaches described in this review are favoured over classical Friedel-Crafts conditions as benzyl-, propargyl- and allyl alcohols, or styrenes, can be used instead of toxic benzyl halides. Additionally, only low catalyst loadings are needed to provide a wide range of products. Following a short introduction about the origin and classical definition of the Friedel-Crafts reaction, the review will describe the different environmentally benign substrates which can be applied today as an approach towards greener processes. Additionally, the first diastereoselective and enantioselective Friedel-Crafts-type alkylations will be highlighted.
Keywords: Friedel–Crafts alkylation; Lewis-acid catalysis; allyl alcohols; arene; asymmetric Friedel–Crafts reaction; benzyl alcohols; green chemistry; hydroalkylation; hydroarylation; propargyl alcohols.
Figures

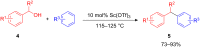
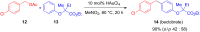
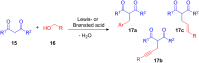
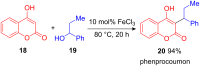



Similar articles
-
A surprising substituent effect provides a superior boronic acid catalyst for mild and metal-free direct Friedel-Crafts alkylations and prenylations of neutral arenes.Chemistry. 2015 Mar 9;21(11):4218-23. doi: 10.1002/chem.201500020. Epub 2015 Feb 10. Chemistry. 2015. PMID: 25678266
-
Unsymmetrical Diarylmethanes by Ferroceniumboronic Acid Catalyzed Direct Friedel-Crafts Reactions with Deactivated Benzylic Alcohols: Enhanced Reactivity due to Ion-Pairing Effects.J Am Chem Soc. 2015 Aug 5;137(30):9694-703. doi: 10.1021/jacs.5b05076. Epub 2015 Jul 23. J Am Chem Soc. 2015. PMID: 26158198
-
Catalytic Enantioselective Friedel-Crafts Allenylation.Angew Chem Int Ed Engl. 2024 Nov 18;63(47):e202413609. doi: 10.1002/anie.202413609. Epub 2024 Oct 14. Angew Chem Int Ed Engl. 2024. PMID: 39108038
-
Beyond Friedel and Crafts: Directed Alkylation of C-H Bonds in Arenes.Angew Chem Int Ed Engl. 2019 May 27;58(22):7202-7236. doi: 10.1002/anie.201806629. Epub 2019 Feb 14. Angew Chem Int Ed Engl. 2019. PMID: 30107097 Review.
-
Beyond Friedel and Crafts: Innate Alkylation of C-H Bonds in Arenes.Angew Chem Int Ed Engl. 2019 Jun 3;58(23):7558-7598. doi: 10.1002/anie.201806631. Epub 2019 Apr 4. Angew Chem Int Ed Engl. 2019. PMID: 30107077 Review.
Cited by
-
Friedel-Crafts acylation of benzene derivatives in tunable aryl alkyl ionic liquids (TAAILs).Beilstein J Org Chem. 2023 Feb 23;19:212-216. doi: 10.3762/bjoc.19.20. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 36865025 Free PMC article.
-
Synergistic Brønsted/Lewis acid catalyzed aromatic alkylation with unactivated tertiary alcohols or di-tert-butylperoxide to synthesize quaternary carbon centers.Chem Sci. 2022 Mar 8;13(12):3539-3548. doi: 10.1039/d1sc06422c. eCollection 2022 Mar 24. Chem Sci. 2022. PMID: 35432882 Free PMC article.
-
Discovery and Optimization of Narrow Spectrum Inhibitors of Tousled Like Kinase 2 (TLK2) Using Quantitative Structure Activity Relationships.bioRxiv [Preprint]. 2023 Dec 28:2023.12.28.573261. doi: 10.1101/2023.12.28.573261. bioRxiv. 2023. Update in: Eur J Med Chem. 2024 May 5;271:116357. doi: 10.1016/j.ejmech.2024.116357. PMID: 38234837 Free PMC article. Updated. Preprint.
-
Recent Development of Bis-Cyclometalated Chiral-at-Iridium and Rhodium Complexes for Asymmetric Catalysis.ACS Org Inorg Au. 2021 Dec 3;2(2):99-125. doi: 10.1021/acsorginorgau.1c00032. eCollection 2022 Apr 6. ACS Org Inorg Au. 2021. PMID: 36855455 Free PMC article. Review.
-
Synthetically important ring opening reactions by alkoxybenzenes and alkoxynaphthalenes.RSC Adv. 2020 Aug 25;10(52):31363-31376. doi: 10.1039/d0ra05111j. eCollection 2020 Aug 21. RSC Adv. 2020. PMID: 35520658 Free PMC article. Review.
References
-
- Friedel C, Crafts J M. J Chem Soc. 1877;32:725–791. doi: 10.1039/JS8773200725. - DOI
-
- Yamauchi T, Hattori K, Mizutaki S, Tamaki K, Uemura S. Bull Chem Soc Jpn. 1986;59:3617–3620. doi: 10.1246/bcsj.59.3617. - DOI
-
- Tsuchimoto T, Tobita K, Hiyama T, Fukuzawa S-i. Synlett. 1996:557–559. doi: 10.1055/s-1996-5498. - DOI
-
- Shimizu I, Khien K M, Nagatomo M, Nakajima T, Yamamoto A. Chem Lett. 1997:851–852. doi: 10.1246/cl.1997.851. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
