Synthesis of indolo[3,2-b]carbazole-based new colorimetric receptor for anions: A unique color change for fluoride ions
- PMID: 20485594
- PMCID: PMC2871001
- DOI: 10.3762/bjoc.6.12
Synthesis of indolo[3,2-b]carbazole-based new colorimetric receptor for anions: A unique color change for fluoride ions
Abstract
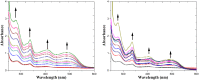
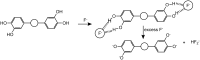
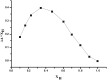
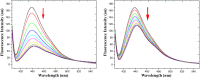
A novel indolocarbazole-based chemosensor 1 containing hydrogen bond donor moieties has been established as a selective colorimetric and fluorometric sensor for F⁻ in CH₃CN/H₂O (4:1 v/v). Upon the addition of a series of tetrabutylammonium salts to receptor 1 in aqueous CH₃CN, only when the counter ion was F⁻ was a significant color change (from light violet to dark orange) observed.
Keywords: anion binding; colorimetry; fluorescence quenching; fluoride binding; indolocarbazole.
Figures






Similar articles
-
Design and synthesis of malonohydrazide based colorimetric receptors for discrimination of maleate over fumarate and detection of F-, AcO- and AsO2- ions.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Mar 15;229:117883. doi: 10.1016/j.saa.2019.117883. Epub 2019 Dec 2. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 31818641
-
A coumarin based Schiff Base: An effective colorimetric sensor for selective detection of F- ion in real samples and DFT studies.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Feb 5;286:121964. doi: 10.1016/j.saa.2022.121964. Epub 2022 Oct 13. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 36274537
-
The example of calix[4]pyrrole derivative containing Bodipy unit: fluorometric and colorimetric sensor for F- ion.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Jan 24;118:903-7. doi: 10.1016/j.saa.2013.09.077. Epub 2013 Oct 10. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24161854
-
Hydrogen-Bonded Colorimetric and Fluorescence Chemosensor for Fluoride Anion With High Selectivity and Sensitivity: A Review.Front Chem. 2021 Aug 19;9:666450. doi: 10.3389/fchem.2021.666450. eCollection 2021. Front Chem. 2021. PMID: 34490204 Free PMC article. Review.
-
Development of the Colorimetric and/or Fluorescent Probes for Detecting Fluoride ions in Aqueous Solution.J Fluoresc. 2024 Nov;34(6):2451-2466. doi: 10.1007/s10895-023-03446-2. Epub 2023 Oct 19. J Fluoresc. 2024. PMID: 37856063 Review.
Cited by
-
Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials.Molecules. 2016 Jun 17;21(6):785. doi: 10.3390/molecules21060785. Molecules. 2016. PMID: 27322228 Free PMC article. Review.
-
A multi-colorimetric probe to discriminate between heavy metal cations and anions in DMSO-H2O with high selectivity for Cu2+ and CN-: study of logic functions and its application in real samples.RSC Adv. 2021 Sep 2;11(47):29466-29485. doi: 10.1039/d1ra04734e. eCollection 2021 Sep 1. RSC Adv. 2021. PMID: 35479545 Free PMC article.
-
A reversible and reusable selective chemosensor for fluoride detection using a phenolic OH-containing BODIPY dye by both colorimetric 'naked-eye' and fluorometric modes.J Fluoresc. 2014 Nov;24(6):1757-66. doi: 10.1007/s10895-014-1464-2. Epub 2014 Sep 26. J Fluoresc. 2014. PMID: 25257399
References
LinkOut - more resources
Full Text Sources
