Templated versus non-templated synthesis of benzo-21-crown-7 and the influence of substituents on its complexing properties
- PMID: 20485596
- PMCID: PMC2869786
- DOI: 10.3762/bjoc.6.14
Templated versus non-templated synthesis of benzo-21-crown-7 and the influence of substituents on its complexing properties
Abstract
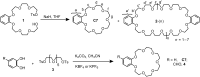
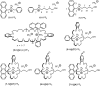
Two procedures for the synthesis of benzo-21-crown-7 have been explored. The [1+1] macrocyclization with KBF₄ as the template was found to be more efficient than the intramolecular macrocyclization without template. Pseudorotaxanes form with secondary ammonium ions bearing at least one alkyl chain narrow enough to slip into the crown ether. Substitution on benzo-21-crown-7 or on the secondary ammonium axle alters the binding affinity and binding mode. Compared to dibenzo-24-crown-8, the complexing properties of benzo-21-crown-7 turn out to be more susceptible to modifications at the crown periphery.
Keywords: benzo-21-crown-7; pseudorotaxane; self-sorting; supramolecular chemistry; template.
Figures



Similar articles
-
Integrative Self-Sorting: One-Pot Synthesis of a Hetero[4]rotaxane from a Daisy-Chain-Containing Hetero[4]pseudorotaxane.Chem Asian J. 2018 Apr 4;13(7):815-821. doi: 10.1002/asia.201800011. Epub 2018 Mar 2. Chem Asian J. 2018. PMID: 29424064
-
[4]Pseudorotaxanes with remarkable self-sorting selectivities.Org Lett. 2011 Sep 2;13(17):4502-5. doi: 10.1021/ol201618f. Epub 2011 Jul 28. Org Lett. 2011. PMID: 21797242
-
[2]Rotaxanes containing pyridinium-phosphonium axles and 24-crown-8 ether wheels.Org Biomol Chem. 2004 Oct 7;2(19):2751-6. doi: 10.1039/B407653B. Epub 2004 Sep 6. Org Biomol Chem. 2004. PMID: 15455146
-
[2]Pseudorotaxane formation between rigid Y-shaped 2,4,5-triphenylimidazolium axles and [24]crown-8 ether wheels.Org Biomol Chem. 2014 Jul 21;12(27):4824-7. doi: 10.1039/c4ob00975d. Org Biomol Chem. 2014. PMID: 24899583
-
Crown Ether-Based Supramolecular Polymers: From Synthesis to Self-Assembly.Macromol Rapid Commun. 2022 Jul;43(14):e2100775. doi: 10.1002/marc.202100775. Epub 2021 Dec 24. Macromol Rapid Commun. 2022. PMID: 34882882 Review.
Cited by
-
Simplicity in the Design, Operation, and Applications of Mechanically Interlocked Molecular Machines.ACS Cent Sci. 2020 Feb 26;6(2):117-128. doi: 10.1021/acscentsci.9b01185. Epub 2020 Jan 22. ACS Cent Sci. 2020. PMID: 32123730 Free PMC article. Review.
-
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes.Beilstein J Org Chem. 2020 Oct 20;16:2576-2588. doi: 10.3762/bjoc.16.209. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 33133289 Free PMC article.
-
Synthesis of multivalent host and guest molecules for the construction of multithreaded diamide pseudorotaxanes.Beilstein J Org Chem. 2012;8:234-245. doi: 10.3762/bjoc.8.24. Epub 2012 Feb 9. Beilstein J Org Chem. 2012. PMID: 22423290 Free PMC article.
-
Responsive macrocyclic and supramolecular structures powered by platinum.Chem Sci. 2023 Dec 11;15(2):431-441. doi: 10.1039/d3sc05524h. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179527 Free PMC article. Review.
-
Self-assembly behaviors of perylene- and naphthalene-crown macrocycle conjugates in aqueous medium.Beilstein J Org Chem. 2019 Jun 3;15:1203-1209. doi: 10.3762/bjoc.15.117. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 31293667 Free PMC article.
References
-
- Sauvage J-P, Dietrich-Buchecker C O, editors. Molecular Catenanes, Rotaxanes and Knots. Weinheim, Germany: Wiley-VCH; 1999.
-
- Stoddart J F, Colquhoun H M. Tetrahedron. 2008;64:8231–8263. doi: 10.1016/j.tet.2008.06.035. - DOI
LinkOut - more resources
Full Text Sources
