Roles of AP-2 in clathrin-mediated endocytosis
- PMID: 20485680
- PMCID: PMC2868873
- DOI: 10.1371/journal.pone.0010597
Roles of AP-2 in clathrin-mediated endocytosis
Abstract
Background: The notion that AP-2 clathrin adaptor is an essential component of an endocytic clathrin coat appears to conflict with recent observations that substantial AP-2 depletion, using RNA interference with synthesis of AP-2 subunits, fails to block uptake of certain ligands known to internalize through a clathrin-based pathway.
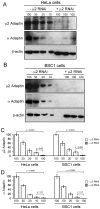
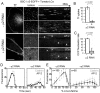
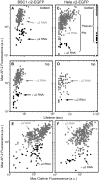
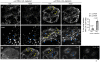
Methodology/principal findings: We report here the use of in vivo imaging data obtained by spinning-disk confocal microscopy to study the formation of clathrin-coated structures at the plasma membranes of BSC1 and HeLa cells depleted by RNAi of the clathrin adaptor, AP-2. Very few clathrin coats continue to assemble after AP-2 knockdown. Moreover, there is a total absence of clathrin-containing structures completely lacking AP-2 while all the remaining coats still contain a small amount of AP-2. These observations suggest that AP-2 is essential for endocytic coated-pit and coated-vesicle formation. We also find that AP-2 knockdown strongly inhibits light-density lipoprotein (LDL) receptor-mediated endocytosis, as long as cells are maintained in complete serum and at 37 degrees C. If cells are first incubated with LDL at 4 degrees C, followed by warming, there is little or no decrease in LDL uptake with respect to control cells. LDL uptake at 37 degrees C is also not affected in AP-2 depleted cells first deprived of LDL by incubation with either serum-starved or LDL-starved cells for 24 hr. The LDL-deprived cells display a significant increase in endocytic structures enriched on deeply invaginated tubes that contain LDL and we suggest that under this condition of stress, LDL might enter through this alternative pathway.
Conclusions/significance: These results suggest that AP-2 is essential for endocytic clathrin coated-pit and coated-vesicle formation. They also indicate that under normal conditions, functional endocytic clathrin coated pits are required for LDL internalization. We also show that under certain conditions of stress, cells can upregulate alternative endocytic structures with the potential to provide compensatory trafficking pathways.
Conflict of interest statement
Figures



References
-
- Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187–198. - PubMed
-
- Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. - PubMed
-
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

