ISO-1, a macrophage migration inhibitory factor antagonist, inhibits airway remodeling in a murine model of chronic asthma
- PMID: 20485865
- PMCID: PMC2935952
- DOI: 10.2119/molmed.2009.00128
ISO-1, a macrophage migration inhibitory factor antagonist, inhibits airway remodeling in a murine model of chronic asthma
Abstract
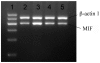
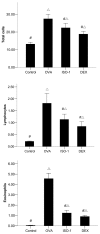
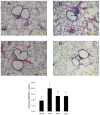
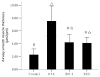
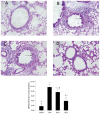
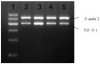
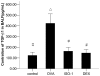
Airway remodeling is the process of airway structural change that occurs in patients with asthma in response to persistent inflammation and leads to increasing disease severity. Drugs that decrease this persistent inflammation play a crucial role in managing asthma episodes. Mice sensitized (by intraperitoneal administration) and then challenged (by inhalation) with ovalbumin (OVA) develop an extensive eosinophilic inflammatory response, goblet cell hyperplasia, collagen deposition, airway smooth muscle thickening, and airway wall area increase, similar to pathologies observed in human asthma. We used OVA-sensitized/challenged mice as a murine model of chronic allergic airway inflammation with subepithelial fibrosis (i.e., asthma). In this OVA mouse model, mRNA and protein of macrophage migration inhibitory factor (MIF) are upregulated, a response similar to what has been observed in the pathogenesis of acute inflammation in human asthma. We hypothesized that MIF induces transforming growth factor-β1 (TGF-β1) synthesis, which has been shown to play an important role in asthma and airway remodeling. To explore the role of MIF in the development of airway remodeling, we evaluated the effects of an MIF small-molecule antagonist, (S,R)3-(4-hy-droxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1), on pathologies associated with the airway-remodeling process in the OVA mouse model. We found that administration of ISO-1 significantly mitigated all symptoms caused by OVA treatment. In addition, the treatment of OVA-sensitized mice with the MIF antagonist ISO-1 significantly reduced TGF-β1 mRNA levels in pulmonary tissue and its protein level in bronchial alveolar lavage fluid supernatants. We believe the repression of MIF in the ISO-1 treatment group led to the significant suppression observed in the inflammatory responses associated with the allergen-induced lung inflammation and fibrosis in our murine asthma (OVA) model. Our results implicate a possible function of MIF in the pathogenesis of chronic asthma and suggest that MIF might be an important therapeutic target for airway remodeling.
Figures


Similar articles
-
MFG-E8/integrin β3 signaling contributes to airway inflammation response and airway remodeling in an ovalbumin-induced murine model of asthma.J Cell Biochem. 2018 Nov;119(11):8887-8896. doi: 10.1002/jcb.27142. Epub 2018 Aug 4. J Cell Biochem. 2018. PMID: 30076648
-
The effects of low dose leukotriene receptor antagonist therapy on airway remodeling and cysteinyl leukotriene expression in a mouse asthma model.Exp Mol Med. 2006 Apr 30;38(2):109-18. doi: 10.1038/emm.2006.14. Exp Mol Med. 2006. PMID: 16672764
-
Inhibition airway remodeling and transforming growth factor-β1/Smad signaling pathway by astragalus extract in asthmatic mice.Int J Mol Med. 2012 Apr;29(4):564-8. doi: 10.3892/ijmm.2011.868. Epub 2011 Dec 23. Int J Mol Med. 2012. Retraction in: Int J Mol Med. 2023 Oct;52(4):87. doi: 10.3892/ijmm.2023.5290. PMID: 22200784 Free PMC article. Retracted.
-
[Advances in pathogenesis of asthma airway remodeling and intervention mechanism of traditional Chinese medicine].Zhongguo Zhong Yao Za Zhi. 2025 Apr;50(8):2050-2070. doi: 10.19540/j.cnki.cjcmm.20241212.705. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40461218 Review. Chinese.
-
Bronchial Asthma, Airway Remodeling and Lung Fibrosis as Successive Steps of One Process.Int J Mol Sci. 2023 Nov 7;24(22):16042. doi: 10.3390/ijms242216042. Int J Mol Sci. 2023. PMID: 38003234 Free PMC article. Review.
Cited by
-
The Role of Macrophage Migration Inhibitory Factor (MIF) in Asthmatic Airway Remodeling.Allergy Asthma Immunol Res. 2021 Jan;13(1):88-105. doi: 10.4168/aair.2021.13.1.88. Allergy Asthma Immunol Res. 2021. PMID: 33191679 Free PMC article.
-
The Role of MIF on Eosinophil Biology and Eosinophilic Inflammation.Clin Rev Allergy Immunol. 2020 Feb;58(1):15-24. doi: 10.1007/s12016-019-08726-z. Clin Rev Allergy Immunol. 2020. PMID: 30680604 Review.
-
The non-mammalian MIF superfamily.Immunobiology. 2017 Mar;222(3):473-482. doi: 10.1016/j.imbio.2016.10.006. Epub 2016 Oct 12. Immunobiology. 2017. PMID: 27780588 Free PMC article. Review.
-
Inhibition of Macrophage Migration Inhibitory Factor Activity Attenuates Haemorrhagic Shock-Induced Multiple Organ Dysfunction in Rats.Front Immunol. 2022 Apr 6;13:886421. doi: 10.3389/fimmu.2022.886421. eCollection 2022. Front Immunol. 2022. PMID: 35464452 Free PMC article.
-
Macrophage migration inhibitory factor exacerbates asthmatic airway remodeling via dynamin-related protein 1-mediated autophagy activation.Respir Res. 2023 Sep 6;24(1):216. doi: 10.1186/s12931-023-02526-y. Respir Res. 2023. PMID: 37674165 Free PMC article.
References
-
- Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–4. - PubMed
-
- Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–21. - PubMed
-
- Wiggs BR, Bosken C, Paré PD, James A, Hogg JC. A model of airway narrowing in asthma and in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1992;145:1251–8. - PubMed
-
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. (Lond) 1998;94:557–72. - PubMed
-
- Boulet LP, et al. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am. J. Respir. Crit. Care Med. 2000;162:1308–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

