Comparison of two methods for carboplatin dosing in children with retinoblastoma
- PMID: 20486170
- PMCID: PMC2921445
- DOI: 10.1002/pbc.22467
Comparison of two methods for carboplatin dosing in children with retinoblastoma
Abstract
Background: Carboplatin is the most effective drug in retinoblastoma but systemic clearance is variable in young patients. While most regimens use a flat dose, individualized targeting may provide a more adjusted systemic exposure.
Patients and methods: We compared carboplatin doses between two groups of children with retinoblastoma that were treated using a flat dose of 560 mg/m(2) or a targeted AUC of 6.5 using a modified Calvert formula.
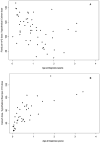
Results: Ninety-eight patients with retinoblastoma received a total of 576 cycles of carboplatin (median 8 cycles). Fifty patients (51%) received a fixed dose per m(2), 32 (33%) received a dose based on AUC, 1 patient received fixed dose per kilogram, and in 15 patients a combination AUC and fixed doses was used. The median cumulative carboplatin dose (mg/m(2)) for patients who received eight cycles using fixed per m(2) dosing was 2151.8 (range, 1414.2-2852.0), compared to 1104.1 for nine patients who received eight cycles using Calvert dosing (range, 779.0-1992.7) (P < 0.001). For cycles given using AUC, the median percentage of the hypothetical fixed per m(2) dose was 70% (range, 48-134%). Younger patients had larger differences. Patients receiving carboplatin based on fixed per m(2) dosing were 3.0 times more likely to have a platelet transfusion (95% confidence interval, 1.3-7.3).
Conclusions: Carboplatin administration needs to consider the changes in renal function occurring during the first months of life. The use of a targeted AUC provides the most accurate method; however, mg per kg of body weight dosing is a very reliable alternative method.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy.Pediatr Blood Cancer. 2008 Feb;50(2):223-6. doi: 10.1002/pbc.21155. Pediatr Blood Cancer. 2008. PMID: 17278120
-
Utility of carboplatin therapeutic drug monitoring for the treatment of neonate and infant retinoblastoma patients in the United Kingdom.Br J Cancer. 2024 Aug;131(3):491-497. doi: 10.1038/s41416-024-02728-1. Epub 2024 Jun 13. Br J Cancer. 2024. PMID: 38871807 Free PMC article.
-
A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma.Ophthalmology. 1999 Oct;106(10):1947-50. doi: 10.1016/S0161-6420(99)90406-2. Ophthalmology. 1999. PMID: 10519590 Clinical Trial.
-
Assessment of hearing in very young children receiving carboplatin for retinoblastoma.Eur J Cancer. 2006 Mar;42(4):492-500. doi: 10.1016/j.ejca.2005.11.004. Epub 2006 Jan 11. Eur J Cancer. 2006. PMID: 16376542 Review.
-
Phase I. Trial of irinotecan plus carboplatin in two dose schedules.Oncology (Williston Park). 2003 May;17(5 Suppl 5):36-40. Oncology (Williston Park). 2003. PMID: 12800605 Review.
Cited by
-
Carboplatin Dosing in Children Using Estimated Glomerular Filtration Rate: Equation Matters.Cancers (Basel). 2021 Nov 26;13(23):5963. doi: 10.3390/cancers13235963. Cancers (Basel). 2021. PMID: 34885072 Free PMC article.
-
Lessons from Retinoblastoma: Implications for Cancer, Development, Evolution, and Regenerative Medicine.Trends Mol Med. 2016 Oct;22(10):863-876. doi: 10.1016/j.molmed.2016.07.010. Epub 2016 Aug 23. Trends Mol Med. 2016. PMID: 27567287 Free PMC article. Review.
-
Model-Informed Precision Dosing of Antibiotics in Pediatric Patients: A Narrative Review.Front Pediatr. 2021 Feb 23;9:624639. doi: 10.3389/fped.2021.624639. eCollection 2021. Front Pediatr. 2021. PMID: 33708753 Free PMC article. Review.
-
Carboplatin-associated ototoxicity in children with retinoblastoma.J Clin Oncol. 2012 Apr 1;30(10):1034-41. doi: 10.1200/JCO.2011.36.9744. Epub 2012 Feb 27. J Clin Oncol. 2012. PMID: 22370329 Free PMC article.
-
Generation of evidence-based carboplatin dosing guidelines for neonates and infants.Br J Cancer. 2023 Nov;129(11):1773-1779. doi: 10.1038/s41416-023-02456-y. Epub 2023 Oct 10. Br J Cancer. 2023. PMID: 37816842 Free PMC article.
References
-
- Young JL, Smith MA, Roffers SD, Liff JM, Bunin GR. Retinoblastoma. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute; Bethesda, MD: 1999. SEER Program. NIH Pub. No. 99–4649.
-
- Rodriguez-Galindo C, Chantada GL, Haik B, Wilson MW. Retinoblastoma: Current treatment and future perspectives. Curr Treat Options Neurol. 2007;9:294–307. - PubMed
-
- Shields CL, Honavar SG, Meadows AT, et al. Chemoreduction plus focal therapy for retinoblastoma: Factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002;133:657–664. - PubMed
-
- Nenadov Beck M, Balmer A, Dessing C, Pica A, Munier F. First-line chemotherapy with local treatment can prevent external-beam irradiation and enucleation in low-stage intraocular retinoblastoma. J Clin Oncol. 2000;18:2881–2887. - PubMed
-
- Wilson MW, Haik BG, Liu T, Merchant TE, Rodriguez-Galindo C. Effect on ocular survivalo of adding early intensive focal treatments to a two-drug chemotherapy regimen in patients with retinoblastoma. Am J Ophthalmol. 2005;140:397–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

