Enhanced long-term transduction and multilineage engraftment of human hematopoietic stem cells transduced with tyrosine-modified recombinant adeno-associated virus serotype 2
- PMID: 20486772
- PMCID: PMC2936497
- DOI: 10.1089/hum.2010.016
Enhanced long-term transduction and multilineage engraftment of human hematopoietic stem cells transduced with tyrosine-modified recombinant adeno-associated virus serotype 2
Abstract
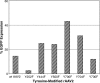
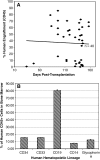
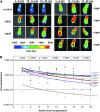
The search for the ideal stem cell gene therapy vector continues as recognized problems persist. Although recombinant adeno-associated virus serotype 2 (rAAV2) mediates gene transfer into hematopoietic stem cells, identified restrictions to transgene expression reduce overall efficiency. Studies have shown that transduction efficiencies are significantly improved by preventing early proteasomal degradation after mutation of surface-exposed tyrosine residues on the capsid to phenylalanine. Here, we report that transduction of human cord blood CD34(+) stem cells by tyrosine-modified rAAV2 is significantly enhanced both in vitro and in vivo. Serial long-term in vivo bioluminescent imaging of immune-deficient recipients after xenotransplantation of CD34(+) cells transduced with tyrosine-modified rAAV2-luciferase revealed that modification of rAAV2 capsids led to a significant increase in the transduction of human CD34(+) cells, without adversely affecting engraftment capacity, or the ability to undergo multilineage differentiation and self-renewal. Together with observations of sustained high-level transgene expression in vivo and efficient persistence of rAAV genomes in human hematopoietic cells, these results suggest that, because of their ability to bypass restrictions to transduction, tyrosine-modified rAAV vectors, particularly Y500F, Y700F, Y444F, and Y704F, represent highly promising candidates for therapeutic evaluation for diseases of human hematopoietic stem cells.
Figures
References
-
- Bainbridge J.W. Smith A.J. Barker S.S. Robbie S. Henderson R. Balaggan K. Viswanathan A. Holder G.E. Stockman A. Tyler N. Petersen-Jones S. Bhattacharya S.S. Thrasher A.J. Fitzke F.W. Carter B.J. Rubin G.S. Moore A.T. Ali R.R. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. - PubMed
-
- Batchu R.B. Shammas M.A. Wang J.Y. Freeman J. Rosen N. Munshi N.C. Adeno-associated virus protects the retinoblastoma family of proteins from adenoviral-induced functional inactivation. Cancer Res. 2002;62:2982–2985. - PubMed
-
- Bell P. Wang L. Lebherz C. Flieder D.B. Bove M.S. Wu D. Gao G.P. Wilson J.M. Wivel N.A. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol. Ther. 2005;12:299–306. - PubMed
-
- Berns K.I. Giraud C. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 1996;218:1–23. - PubMed
-
- Biffi A. Cesani M. Human hematopoietic stem cells in gene therapy: Pre-clinical and clinical issues. Curr. Gene Ther. 2008;8:135–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

