Bone morphogenetic protein 4 mediates human embryonic germ cell derivation
- PMID: 20486775
- PMCID: PMC3128759
- DOI: 10.1089/scd.2010.0084
Bone morphogenetic protein 4 mediates human embryonic germ cell derivation
Abstract
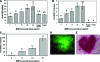
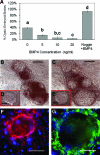
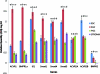
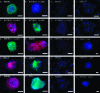
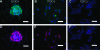
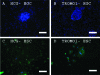
Human primordial germ cells (PGCs) have proven to be a source of pluripotent stem cells called embryonic germ cells (EGCs). Unlike embryonic stem cells, virtually little is known regarding the factors that regulate EGC survival and maintenance. In mice, the growth factor bone morphogenetic protein 4 (BMP4) has been shown to be required for maintaining mouse embryonic stem cells, and disruptions in this gene lead to defects in mouse PGC specification. Here, we sought to determine whether recombinant human BMP4 could influence EGC derivation and/or human PGC survival. We found that the addition of recombinant BMP4 increased the number of human PGCs after 1 week of culture in a dose-responsive manner. The efficiency of EGC derivation and maintenance in culture was also enhanced by the presence of recombinant BMP4 based on alkaline phosphatase and OCT4 staining. In addition, an antagonist of the BMP4 pathway, Noggin, decreased PGC proliferation and led to an increase in cystic embryoid body formation. Quantitative real-time (qRT)-polymerase chain reaction analyses and immunostaining confirmed that the constituents of the BMP4 pathway were upregulated in EGCs versus PGCs. Downstream activators of the BMP4 pathway such as ID1 and phosphorylated SMADs 1 and 5 were also expressed, suggesting a role of this growth factor in EGC pluripotency.
Figures
Similar articles
-
BMP signaling regulates PGC numbers and motility in organ culture.Mech Dev. 2007 Jan;124(1):68-77. doi: 10.1016/j.mod.2006.09.005. Epub 2006 Sep 30. Mech Dev. 2007. PMID: 17112707
-
Influence of activin A supplementation during human embryonic stem cell derivation on germ cell differentiation potential.Stem Cells Dev. 2013 Dec 1;22(23):3141-55. doi: 10.1089/scd.2013.0024. Epub 2013 Aug 14. Stem Cells Dev. 2013. PMID: 23829223 Free PMC article.
-
Bone morphogenetic protein 4 is an efficient inducer for mouse embryonic stem cell differentiation into primordial germ cell.In Vitro Cell Dev Biol Anim. 2011 Jun;47(5-6):391-8. doi: 10.1007/s11626-011-9404-9. Epub 2011 Apr 27. In Vitro Cell Dev Biol Anim. 2011. PMID: 21523484
-
Induction of primordial germ cells from pluripotent epiblast.ScientificWorldJournal. 2002 Mar 26;2:801-10. doi: 10.1100/tsw.2002.155. ScientificWorldJournal. 2002. PMID: 12806005 Free PMC article. Review.
-
In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells.Curr Stem Cell Res Ther. 2009 May;4(2):87-97. doi: 10.2174/157488809788167391. Curr Stem Cell Res Ther. 2009. PMID: 19442193 Review.
Cited by
-
Germline stem cells in human.Signal Transduct Target Ther. 2022 Oct 2;7(1):345. doi: 10.1038/s41392-022-01197-3. Signal Transduct Target Ther. 2022. PMID: 36184610 Free PMC article. Review.
-
Direct Reprogramming of Human Primordial Germ Cells into Induced Pluripotent Stem Cells: Efficient Generation of Genetically Engineered Germ Cells.Stem Cells Dev. 2015 Nov 15;24(22):2634-48. doi: 10.1089/scd.2015.0100. Epub 2015 Aug 10. Stem Cells Dev. 2015. PMID: 26154167 Free PMC article.
-
Primordial germ cell-like cells derived from canine adipose mesenchymal stem cells.Cell Prolif. 2016 Aug;49(4):503-11. doi: 10.1111/cpr.12271. Epub 2016 Jul 4. Cell Prolif. 2016. PMID: 27374854 Free PMC article.
-
The TGF-β Family in the Reproductive Tract.Cold Spring Harb Perspect Biol. 2017 Oct 3;9(10):a022251. doi: 10.1101/cshperspect.a022251. Cold Spring Harb Perspect Biol. 2017. PMID: 28193725 Free PMC article. Review.
-
Genome-wide profiling of pluripotent cells reveals a unique molecular signature of human embryonic germ cells.PLoS One. 2012;7(6):e39088. doi: 10.1371/journal.pone.0039088. Epub 2012 Jun 21. PLoS One. 2012. PMID: 22737227 Free PMC article.
References
-
- Donovan PJ. Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414:92–97. - PubMed
-
- Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. - PubMed
-
- Evans MJ. Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

