Minozac treatment prevents increased seizure susceptibility in a mouse "two-hit" model of closed skull traumatic brain injury and electroconvulsive shock-induced seizures
- PMID: 20486807
- PMCID: PMC2942875
- DOI: 10.1089/neu.2009.1227
Minozac treatment prevents increased seizure susceptibility in a mouse "two-hit" model of closed skull traumatic brain injury and electroconvulsive shock-induced seizures
Abstract
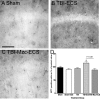
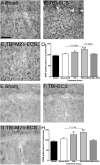
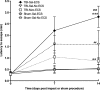
The mechanisms linking traumatic brain injury (TBI) to post-traumatic epilepsy (PTE) are not known and no therapy for prevention of PTE is available. We used a mouse closed-skull midline impact model to test the hypotheses that TBI increases susceptibility to seizures in a "two-hit" injury model, and that suppression of cytokine upregulation after the first hit will attenuate the increased susceptibility to the second neurological insult. Adult male CD-1 mice underwent midline closed skull pneumatic impact. At 3 and 6 h after impact or sham procedure, the mice were injected IP with either Minozac (Mzc), a suppressor of proinflammatory cytokine upregulation, or vehicle (saline). On day 7 after sham operation or TBI, seizures were induced using electroconvulsive shock (ECS), and susceptibility to seizures was measured by the current required for seizure induction. Activation of glia, neuronal injury, and metallothionein-immunoreactive cells were quantified in the hippocampus by immunohistochemical methods. Neurobehavioral function over 14-day recovery was quantified using the Barnes maze. Following TBI there was a significant increase in susceptibility to seizures induced by ECS, and this susceptibility was prevented by suppression of cytokine upregulation with Mzc. Astrocyte activation, metallothionein expression, and neurobehavioral impairment were also increased in the two-hit group subjected to combined TBI and ECS. These enhanced responses in the two-hit group were also prevented by suppression of proinflammatory cytokine upregulation with Mzc. These data implicate glial activation in the mechanisms of epileptogenesis after TBI, and identify a potential therapeutic approach to attenuate the delayed neurological sequelae of TBI.
Figures





References
-
- Ambjørn M. Asmussen J. Lindstam M. Gotfryd K. Jacobsen C. Kiselyov V. Moestrup S. Penkowa M. Bock E. Berezin V. Metallothionein and a peptide modeled after metallothionein, EmtinB, induce neuronal differentiation and survival through binding to receptors of the low-density lipoprotein receptor family. J. Neurochem. 2007;104:21–37. - PubMed
-
- Barnes C. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

