Bacterial protein acetylation: the dawning of a new age
- PMID: 20487279
- PMCID: PMC2907427
- DOI: 10.1111/j.1365-2958.2010.07204.x
Bacterial protein acetylation: the dawning of a new age
Abstract
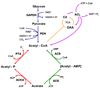
Protein acetylation has historically been considered a predominantly eukaryotic phenomenon. Recent evidence, however, supports the hypothesis that acetylation broadly impacts bacterial physiology. To explore more rapidly the impact of protein acetylation in bacteria, microbiologists can benefit from the strong foundation established by investigators of protein acetylation in eukaryotes. To help advance this learning process, we will summarize the current understanding of protein acetylation in eukaryotes, discuss the emerging link between acetylation and metabolism and highlight the best-studied examples of protein acetylation in bacteria.
Figures


Similar articles
-
Protein lysine acetylation in bacteria: Current state of the art.Proteomics. 2016 Jan;16(2):301-9. doi: 10.1002/pmic.201500258. Epub 2015 Dec 14. Proteomics. 2016. PMID: 26390373 Review.
-
Protein Acetylation and Its Role in Bacterial Virulence.Trends Microbiol. 2017 Sep;25(9):768-779. doi: 10.1016/j.tim.2017.04.001. Epub 2017 Apr 24. Trends Microbiol. 2017. PMID: 28462789 Review.
-
[Prenylation: from bacteria to eukaryotes].Mol Biol (Mosk). 2013 Sep-Oct;47(5):717-30. Mol Biol (Mosk). 2013. PMID: 25509344 Review. Russian.
-
Protein acetylation in archaea, bacteria, and eukaryotes.Archaea. 2010 Sep 16;2010:820681. doi: 10.1155/2010/820681. Archaea. 2010. PMID: 20885971 Free PMC article. Review.
-
Protein Acetylation in Bacteria.Annu Rev Microbiol. 2019 Sep 8;73:111-132. doi: 10.1146/annurev-micro-020518-115526. Epub 2019 May 15. Annu Rev Microbiol. 2019. PMID: 31091420 Free PMC article. Review.
Cited by
-
First Comprehensive Proteome Analyses of Lysine Acetylation and Succinylation in Seedling Leaves of Brachypodium distachyon L.Sci Rep. 2016 Aug 12;6:31576. doi: 10.1038/srep31576. Sci Rep. 2016. PMID: 27515067 Free PMC article.
-
Glycolysis for Microbiome Generation.Microbiol Spectr. 2015 Jun;3(3):10.1128/microbiolspec.MBP-0014-2014. doi: 10.1128/microbiolspec.MBP-0014-2014. Microbiol Spectr. 2015. PMID: 26185089 Free PMC article.
-
Characterizing the Effect of the Lysine Deacetylation Modification on Enzyme Activity of Pyruvate Kinase I and Pathogenicity of Vibrio alginolyticus.Front Vet Sci. 2022 Jun 6;9:877067. doi: 10.3389/fvets.2022.877067. eCollection 2022. Front Vet Sci. 2022. PMID: 35795782 Free PMC article.
-
Antifragility and Tinkering in Biology (and in Business) Flexibility Provides an Efficient Epigenetic Way to Manage Risk.Genes (Basel). 2011 Nov 29;2(4):998-1016. doi: 10.3390/genes2040998. Genes (Basel). 2011. PMID: 24710302 Free PMC article.
-
Acetate availability and utilization supports the growth of mutant sub-populations on aging bacterial colonies.PLoS One. 2014 Oct 2;9(10):e109255. doi: 10.1371/journal.pone.0109255. eCollection 2014. PLoS One. 2014. PMID: 25275605 Free PMC article.
References
-
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. - PubMed
-
- Barak R, Eisenbach M. Acetylation of the response regulator, CheY, is involved in bacterial chemotaxis. Mol Microbiol. 2001;40:731–743. - PubMed
-
- Barak R, Prasad K, Shainskaya A, Wolfe AJ, Eisenbach M. Acetylation of the chemotaxis response regulator CheY by acetyl-CoA synthetase purified from Escherichia coli. J Mol Biol. 2004;342:383–401. - PubMed
-
- Barak R, Welch M, Yanovsky A, Oosawa K, Eisenbach M. Acetyladenylate or its derivative acetylates the chemotaxis protein CheY in vitro and increases its activity at the flagellar switch. Biochemistry. 1992;31:10099–10107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

