Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV
- PMID: 20487288
- PMCID: PMC3124366
- DOI: 10.1111/j.1365-2958.2009.07027.x
Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV
Erratum in
- Mol Microbiol. 2010 Mar;75(5):1325
Abstract
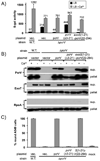
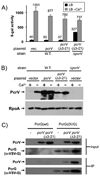
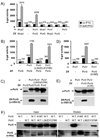
Pseudomonas aeruginosa uses a type III secretion system to inject protein effectors into a targeted host cell. Effector secretion is triggered by host cell contact. How effector secretion is prevented prior to cell contact is not well understood. In all secretion systems studied to date, the needle tip protein is required for controlling effector secretion, but the mechanism by which needle tip proteins control effector secretion is unclear. Here we present data that the P. aeruginosa needle tip protein, PcrV, controls effector secretion by assembling into a functional needle tip complex. PcrV likely does not simply obstruct the secretion channel because the pore-forming translocator proteins can still be secreted while effector secretion is repressed. This finding suggests that PcrV controls effector secretion by affecting the conformation of the apparatus, shifting it from the default, effector secretion 'on' conformation, to the effector secretion 'off' conformation. We also present evidence that PcrG, which can bind to PcrV and is also involved in controlling effector export, is cytoplasmic and that the interaction between PcrG and PcrV is not required for effector secretion control by either protein. Taken together, these data allow us to propose a working model for control of effector secretion by PcrG and PcrV.
Figures






Similar articles
-
Type III secretion proteins PcrV and PcrG from Pseudomonas aeruginosa form a 1:1 complex through high affinity interactions.BMC Microbiol. 2003 Oct 18;3:21. doi: 10.1186/1471-2180-3-21. BMC Microbiol. 2003. PMID: 14565848 Free PMC article.
-
PcrG protects the two long helical oligomerization domains of PcrV, by an interaction mediated by the intramolecular coiled-coil region of PcrG.BMC Struct Biol. 2014 Jan 24;14:5. doi: 10.1186/1472-6807-14-5. BMC Struct Biol. 2014. PMID: 24460624 Free PMC article.
-
Modified needle-tip PcrV proteins reveal distinct phenotypes relevant to the control of type III secretion and intoxication by Pseudomonas aeruginosa.PLoS One. 2011 Mar 29;6(3):e18356. doi: 10.1371/journal.pone.0018356. PLoS One. 2011. PMID: 21479247 Free PMC article.
-
The type III secretion system of Pseudomonas aeruginosa: infection by injection.Nat Rev Microbiol. 2009 Sep;7(9):654-65. doi: 10.1038/nrmicro2199. Nat Rev Microbiol. 2009. PMID: 19680249 Free PMC article. Review.
-
Structure and biophysics of type III secretion in bacteria.Biochemistry. 2013 Apr 16;52(15):2508-17. doi: 10.1021/bi400160a. Epub 2013 Apr 5. Biochemistry. 2013. PMID: 23521714 Free PMC article. Review.
Cited by
-
Calcium chelation by alginate activates the type III secretion system in mucoid Pseudomonas aeruginosa biofilms.PLoS One. 2012;7(10):e46826. doi: 10.1371/journal.pone.0046826. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056471 Free PMC article.
-
Dimerization of the Pseudomonas aeruginosa translocator chaperone PcrH is required for stability, not function.J Bacteriol. 2013 Nov;195(21):4836-43. doi: 10.1128/JB.00335-13. Epub 2013 Aug 23. J Bacteriol. 2013. PMID: 23974025 Free PMC article.
-
Full Transcriptomic Response of Pseudomonas aeruginosa to an Inulin-Derived Fructooligosaccharide.Front Microbiol. 2020 Feb 20;11:202. doi: 10.3389/fmicb.2020.00202. eCollection 2020. Front Microbiol. 2020. PMID: 32153524 Free PMC article.
-
Self-assembled ferritin nanoparticles displaying PcrV and OprI as an adjuvant-free Pseudomonas aeruginosa vaccine.Front Immunol. 2023 Jun 21;14:1184863. doi: 10.3389/fimmu.2023.1184863. eCollection 2023. Front Immunol. 2023. PMID: 37415986 Free PMC article.
-
Functional and Structural Characterization of OXA-935, a Novel OXA-10-Family β-Lactamase from Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2022 Oct 18;66(10):e0098522. doi: 10.1128/aac.00985-22. Epub 2022 Sep 21. Antimicrob Agents Chemother. 2022. PMID: 36129295 Free PMC article.
References
-
- Botteaux A, Sory MP, Biskri L, Parsot C, Allaoui A. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol Microbiol. 2008 - PubMed
-
- Broms JE, Forslund AL, Forsberg A, Francis MS. PcrH of Pseudomonas aeruginosa is essential for secretion and assembly of the type III translocon. J Infect Dis. 2003;188:1909–1921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

