Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of Hedgehog signal transduction on primary cilia in the zebrafish
- PMID: 20487519
- PMCID: PMC2890509
- DOI: 10.1186/1741-7007-8-65
Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of Hedgehog signal transduction on primary cilia in the zebrafish
Abstract
Background: In mammalian cells, the integrity of the primary cilium is critical for proper regulation of the Hedgehog (Hh) signal transduction pathway. Whether or not this dependence on the primary cilium is a universal feature of vertebrate Hedgehog signalling has remained contentious due, in part, to the apparent divergence of the intracellular transduction pathway between mammals and teleost fish.
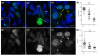
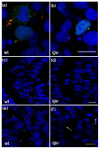
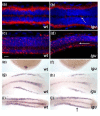
Results: Here, using a functional Gli2-GFP fusion protein, we show that, as in mammals, the Gli2 transcription factor localizes to the primary cilia of cells in the zebrafish embryo and that this localization is modulated by the activity of the Hh pathway. Moreover, we show that the Igu/DZIP1protein, previously implicated in the modulation of Gli activity in zebrafish, also localizes to the primary cilium and is required for its proper formation.
Conclusion: Our findings demonstrate a conserved role of the primary cilium in mediating Hedgehog signalling activity across the vertebrate phylum and validate the use of the zebrafish as a representative model for the in vivo analysis of vertebrate Hedgehog signalling.
Figures




Comment in
-
Vertebrate Hedgehog signaling: cilia rule.BMC Biol. 2010 Jul 29;8:102. doi: 10.1186/1741-7007-8-102. BMC Biol. 2010. PMID: 20687907 Free PMC article.
References
-
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. full_text. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

