TRial of an Educational intervention on patients' knowledge of Atrial fibrillation and anticoagulant therapy, INR control, and outcome of Treatment with warfarin (TREAT)
- PMID: 20487528
- PMCID: PMC2887377
- DOI: 10.1186/1471-2261-10-21
TRial of an Educational intervention on patients' knowledge of Atrial fibrillation and anticoagulant therapy, INR control, and outcome of Treatment with warfarin (TREAT)
Abstract
Background: Atrial fibrillation (AF) patients with a high risk of stroke are recommended anticoagulation with warfarin. However, the benefit of warfarin is dependent upon time spent within the target therapeutic range (TTR) of their international normalised ratio (INR) (2.0 to 3.0). AF patients possess limited knowledge of their disease and warfarin treatment and this can impact on INR control. Education can improve patients' understanding of warfarin therapy and factors which affect INR control.
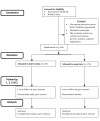
Methods/design: Randomised controlled trial of an intensive educational intervention will consist of group sessions (between 2-8 patients) containing standardised information about the risks and benefits associated with OAC therapy, lifestyle interactions and the importance of monitoring and control of their International Normalised Ratio (INR). Information will be presented within an 'expert-patient' focussed DVD, revised educational booklet and patient worksheets. 200 warfarin-naïve patients who are eligible for warfarin will be randomised to either the intervention or usual care groups. All patients must have ECG-documented AF and be eligible for warfarin (according to the NICE AF guidelines). Exclusion criteria include: aged < 18 years old, contraindication(s) to warfarin, history of warfarin USE, valvular heart disease, cognitive impairment, are unable to speak/read English and disease likely to cause death within 12 months. Primary endpoint is time spent in TTR. Secondary endpoints include measures of quality of life (AF-QoL-18), anxiety and depression (HADS), knowledge of AF and anticoagulation, beliefs about medication (BMQ) and illness representations (IPQ-R). Clinical outcomes, including bleeding, stroke and interruption to anticoagulation will be recorded. All outcome measures will be assessed at baseline and 1, 2, 6 and 12 months post-intervention.
Discussion: More data is needed on the clinical benefit of educational intervention with AF patients receiving warfarin.
Trial registration: ISRCTN93952605.
References
-
- Lip GYH, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: A systematic review and meta-analysis. Thrombo Res. 2006;110:354–358. - PubMed
-
- Hart RG, Pearce LA, Aguillar ML. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous