Clinical and serological evaluation of a novel CENP-A peptide based ELISA
- PMID: 20487535
- PMCID: PMC2911886
- DOI: 10.1186/ar3029
Clinical and serological evaluation of a novel CENP-A peptide based ELISA
Abstract
Introduction: Anti-centromere antibodies (ACA) are useful biomarkers in the diagnosis of systemic sclerosis (SSc). ACA are found in 20 to 40% of SSc patients and, albeit with lower prevalence, in patients with other systemic autoimmune rheumatic diseases. Historically, ACA were detected by indirect immunofluorescence (IIF) on HEp-2 cells and confirmed by immunoassays using recombinant CENP-B. The objective of this study was to evaluate a novel CENP-A peptide ELISA.
Methods: Sera collected from SSc patients (n=334) and various other diseases (n=619) and from healthy controls (n=175) were tested for anti-CENP-A antibodies by the novel CENP-A enzyme linked immunosorbent assay (ELISA). Furthermore, ACA were determined in the disease cohorts by IIF (ImmunoConcepts, Sacramento, CA, USA), CENP-B ELISA (Dr. Fooke), EliA CENP (Phadia, Freiburg, Germany) and line-immunoassay (LIA, Mikrogen, Neuried, Germany). Serological and clinical associations of anti-CENP-A with other autoantibodies were conducted in one participating centre. Inhibition experiments with either the CENP-A peptide or recombinant CENP-B were carried out to analyse the specificity of anti-CENP-A and -B antibodies.
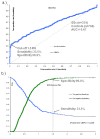
Results: The CENP-A ELISA results were in good agreement with other ACA detection methods. According to the kappa method, the qualitative agreements were: 0.73 (vs. IIF), 0.81 (vs. LIA), 0.86 (vs. CENP-B ELISA) and 0.97 (vs. EliA CENP). The quantitative comparison between CENP-A and CENP-B ELISA using 265 samples revealed a correlation value of rho=0.5 (by Spearman equation). The receiver operating characteristic analysis indicated that the discrimination between SSc patients (n=131) and various controls (n=134) was significantly better using the CENP-A as compared to CENP-B ELISA (P<0.0001). Modified Rodnan skin score was significantly lower in the CENP-A negative group compared to the positive patients (P=0.013). Inhibition experiments revealed no significant cross reactivity of anti-CENP-A and anti-CENP-B antibodies. Statistically relevant differences for gender ratio (P=0.0103), specific joint involvement (Jaccoud) (P=0.0006) and anti-phospholipid syndrome (P=0.0157) between ACA positive SLE patients and the entire SLE cohort were observed.
Conclusions: Anti-CENP-A antibodies as determined by peptide ELISA represent a sensitive, specific and independent marker for the detection of ACA and are useful biomarkers for the diagnosis of SSc. Our data suggest that anti-CENP-A antibodies are a more specific biomarker for SSc than antibodies to CENP-B. Furthers studies are required to verify these findings.
Figures

References
-
- Miyawaki S, Asanuma H, Nishiyama S, Yoshinaga Y. Clinical and serological heterogeneity in patients with anticentromere antibodies. J Rheumatol. 2005;32:1488–1494. - PubMed
-
- Tojo J, Ohira H, Suzuki T, Takeda I, Shoji I, Kojima T, Takagi T, Kuroda M, Miyata M, Nishimaki T, Ohnami K, Kanno T, Mukai S, Kasukawa R. Clinicolaboratory characteristics of patients with primary biliary cirrhosis associated with CREST symptoms. Hepatol Res. 2002;22:187–195. doi: 10.1016/S1386-6346(01)00138-3. - DOI - PubMed
-
- Assassi S, Fritzler MJ, Arnett FC, Norman GL, Shah KR, Gourh P, Manek N, Perry M, Ganesh D, Rahbar MH, Mayes MD. Primary biliary cirrhosis (PBC), PBC autoantibodies, and hepatic parameter abnormalities in a large population of systemic sclerosis patients. J Rheumatol. 2009;36:2250–2256. doi: 10.3899/jrheum.090340. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

