Beta-escin has potent anti-allergic efficacy and reduces allergic airway inflammation
- PMID: 20487574
- PMCID: PMC2898835
- DOI: 10.1186/1471-2172-11-24
Beta-escin has potent anti-allergic efficacy and reduces allergic airway inflammation
Abstract
Background: Type I hypersensitivity is characterized by the overreaction of the immune system against otherwise innocuous substances. It manifests as allergic rhinitis, allergic conjunctivitis, allergic asthma or atopic dermatitis if mast cells are activated in the respective organs. In case of systemic mast cell activation, life-threatening anaphylaxis may occur. Currently, type I hypersensitivities are treated either with glucocorticoids, anti-histamines, or mast cell stabilizers. Although these drugs exert a strong anti-allergic effect, their long-term use may be problematic due to their side-effects.
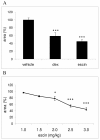
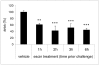
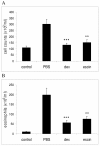
Results: In the course of a routine in vitro screening process, we identified beta-escin as a potentially anti-allergic compound. Here we tested beta-escin in two mouse models to confirm this anti-allergic effect in vivo. In a model of the early phase of allergic reactions, the murine passive cutaneous anaphylaxis model, beta-escin inhibited the effects of mast cell activation and degranulation in the skin and dose-dependently prevented the extravasation of fluids into the tissue. Beta-escin also significantly inhibited the late response after antigen challenge in a lung allergy model with ovalbumin-sensitized mice. Allergic airway inflammation was suppressed, which was exemplified by the reduction of leucocytes, eosinophils, IL-5 and IL-13 in the bronchoalveolar lavage fluid. Histopathological examinations further confirmed the reduced inflammation of the lung tissue. In both models, the inhibitory effect of beta-escin was comparable to the benchmark dexamethasone.
Conclusions: We demonstrated in two independent murine models of type I hypersensitivity that beta-escin has potent anti-allergic properties. These results and the excellent safety profile of beta-escin suggest a therapeutic potential of this compound for a novel treatment of allergic diseases.
Figures


References
-
- Baumruker T, Prieschl E. Mast cells and their activation - from the molecular mechanism to clinical relevance. Mod Asp Immunobiol. 2001;1:259–262.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

