The efficiency of selenocysteine incorporation is regulated by translation initiation factors
- PMID: 20488192
- PMCID: PMC3721751
- DOI: 10.1016/j.jmb.2010.05.026
The efficiency of selenocysteine incorporation is regulated by translation initiation factors
Abstract
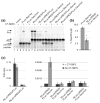
Selenocysteine (Sec) incorporation is an essential process required for the production of at least 25 human selenoproteins. This unique amino acid is co-translationally incorporated at specific UGA codons that normally serve as termination signals. Recoding from stop to Sec involves a cis-acting Sec insertion sequence element in the 3' untranslated region of selenoprotein mRNAs as well as Sec insertion sequence binding protein 2, Sec-tRNA(Sec), and the Sec-specific elongation factor, eEFSec. The interplay between recoding and termination at Sec codons has served as a focal point in researching the mechanism of Sec insertion, but the role of translation initiation has not been addressed. In this report, we show that the cricket paralysis virus intergenic internal ribosome entry site is able to support Sec incorporation, thus providing evidence that the canonical functions of translation initiation factors are not required. Additionally, we show that neither a 5' cap nor a 3' poly(A) tail enhances Sec incorporation. Interestingly, however, the presence of the internal ribosome entry site significantly decreases Sec incorporation efficiency, suggesting a role for translation initiation in regulating the efficiency of UGA recoding.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Allmang C, Krol A. Selenoprotein synthesis: UGA does not end the story. Biochimie. 2006;88:1561–1571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

