Animal models of choroidal and retinal neovascularization
- PMID: 20488255
- PMCID: PMC2962694
- DOI: 10.1016/j.preteyeres.2010.05.003
Animal models of choroidal and retinal neovascularization
Abstract
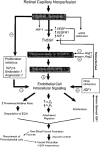
There have been numerous types of animal models of choroidal neovascularization (CNV) and retinal neovascularization (RNV). Understanding the pathobiology of CNV and RNV is important when evaluating and utilizing these models. Both CNV and RNV are dynamic processes. A break or defect in Bruchs' membrane is necessary for CNV to develop. This may be induced with a laser, mechanically via surgery, or in the setting of transgenic mice. Some of the transgenic mouse models spontaneously develop RNV and/or retinal angiomatous proliferation (RAP)-like lesions. The pathogenesis of RNV is well-known and is generally related to ischemic retinopathy. Models of oxygen-induced retinopathy (OIR) closely resemble retinopathy of prematurity (ROP). The streptozotocin (STZ) rat model develops features similar to diabetic retinopathy. This review summarizes general categories and specific examples of animal models of CNV and RNV. There are no perfect models of CNV or RNV and individual investigators are encouraged to choose the model that best suits their needs.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
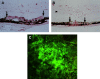
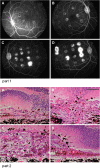

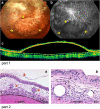
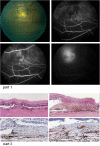






References
-
- Aguilar E, Dorrell MI, Friedlander D, Jacobson RA, Johnson A, Marchetti V, Moreno SK, Ritter MR, Friedlander M. Ocular models of angiogenesis. Methods Enzymol. 2008;444:115–158. - PubMed
-
- Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2- deficient mice. Nat Med. 2003;11:1290–1397. - PubMed
-
- Amin R, Pucklin JE, et al. Growth factor localization in choroidal noevascular membranes of age-related macular degeneration. Inv Ophthalmol Vis Sci. 1994;35:3178–3188. - PubMed
-
- Antoszyk AN, Gottlieb JL, Machemer R, Hatchell DL. The effects of intravitreal triamcinolone acetonide on experimental pre-retinal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1993;231:34–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

