Molecular characterization of genome segment 2 encoding RNA dependent RNA polymerase of Antheraea mylitta cytoplasmic polyhedrosis virus
- PMID: 20488502
- PMCID: PMC7111928
- DOI: 10.1016/j.virol.2010.04.019
Molecular characterization of genome segment 2 encoding RNA dependent RNA polymerase of Antheraea mylitta cytoplasmic polyhedrosis virus
Abstract
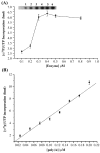
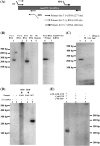
Genome segment 2 (S2) from Antheraea mylitta cypovirus (AmCPV) was converted into cDNA, cloned and sequenced. S2 consisted of 3798 nucleotides with a long ORF encoding a 1116 amino acid long protein (123 kDa). BLAST and phylogenetic analysis showed 29% sequence identity and close relatedness of AmCPV S2 with RNA dependent RNA polymerase (RdRp) of other insect cypoviruses, suggesting a common origin of all insect cypoviruses. The ORF of S2 was expressed as 123 kDa soluble His-tagged fusion protein in insect cells via baculovirus recombinants which exhibited RdRp activity in an in vitro RNA polymerase assay without any intrinsic terminal transferase activity. Maximum activity was observed at 37 degrees C at pH 6.0 in the presence of 3 mM MgCl(2). Site directed mutagenesis confirmed the importance of the conserved GDD motif. This is the first report of functional characterization of a cypoviral RdRp which may lead to the development of anti-viral agents.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures





References
-
- Attoui H., Billoir F., Biagini P., Cantaloube J.F., Chesse R.D., Micco P.D., Lamballerie X.D. Sequence determination and analysis of the full-length genome of Colorado tick fever virus, the type species of genus coltivirus (Family Reoviridae) Biochem. Biophys. Res. Commun. 2000;273:1121–1125. - PubMed
-
- Attoui H., Jaafar F.M., Mertens P.P.C., Lamballerie X.D. Seadornavirus. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; Amsterdam: 2005.
-
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith A.J., Struhl K., editors. Current Protocols in Molecular Biology. John Wiley and Sons, Inc; New York: 1995.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

