Amelioration of nephropathy with apoA-1 mimetic peptide in apoE-deficient mice
- PMID: 20488818
- PMCID: PMC2980997
- DOI: 10.1093/ndt/gfq274
Amelioration of nephropathy with apoA-1 mimetic peptide in apoE-deficient mice
Abstract
Background: There is mounting evidence that dyslipidaemia may contribute to development and progression of renal disease. For instance, hyperlipidaemia in apolipoprotein E-deficient (apoE(-/-)) mice is associated with glomerular inflammation, mesangial expansion and foam cell formation. ApoA-1 mimetic peptides are potent antioxidant and anti-inflammatory compounds which are highly effective in ameliorating atherosclerosis and inflammation in experimental animals. Given the central role of oxidative stress and inflammation in progression of renal disease, we hypothesized that apoA-1 mimetic peptide, D-4F, may attenuate renal lesions in apoE(-/-) mice.
Methods: Twenty-five-month-old female apoE(-/-) mice were treated with D-4F (300 µg/mL in drinking water) or placebo for 6 weeks. Kidneys were harvested and examined for histological and biochemical characteristics.
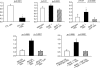
Results: Compared with the control mice, apoE(-/-) mice showed significant proteinuria, tubulo-interstitial inflammation, mesangial expansion, foam cell formation and up-regulation of oxidative [NAD(P)H oxidase subunits] and inflammatory [NF-κB, MCP-1, PAI-1 and COX-2] pathways. D-4F administration lowered proteinuria, improved renal histology and reversed up-regulation of inflammatory and oxidative pathways with only minimal changes in plasma lipid levels.
Conclusions: The apoE(-/-) mice develop proteinuria and glomerular and tubulo-interstitial injury which are associated with up-regulation of oxidative and inflammatory mediators in the kidney and are ameliorated by the administration of apoA-1 mimetic peptide. These observations point to the role of oxidative stress and inflammation in the pathogenesis of renal disease in hyperlipidaemic animals and perhaps humans.
Figures






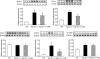
Similar articles
-
ApoA-1 mimetic peptide reverses uremia-induced upregulation of pro-atherogenic pathways in the aorta.Am J Nephrol. 2010;32(3):201-211. doi: 10.1159/000316479. Epub 2010 Jul 16. Am J Nephrol. 2010. PMID: 20639628
-
Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes.Diabetes. 2010 Dec;59(12):3223-8. doi: 10.2337/db10-0844. Epub 2010 Sep 8. Diabetes. 2010. PMID: 20826564 Free PMC article.
-
Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice.Circulation. 2004 Jun 29;109(25):3215-20. doi: 10.1161/01.CIR.0000134275.90823.87. Epub 2004 Jun 14. Circulation. 2004. PMID: 15197147
-
Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis.Cardiol Rev. 2010 May-Jun;18(3):141-7. doi: 10.1097/CRD.0b013e3181c4b508. Cardiol Rev. 2010. PMID: 20395699 Review.
-
Apolipoprotein A-I mimetic peptides.Curr Atheroscler Rep. 2009 Jan;11(1):52-7. doi: 10.1007/s11883-009-0008-8. Curr Atheroscler Rep. 2009. PMID: 19080728 Free PMC article. Review.
Cited by
-
ApoE deficiency promotes colon inflammation and enhances inflammatory potential oxidized-LDL and TNF-α in colon epithelial cells.Biosci Rep. 2016 Oct;36(5):e00388. doi: 10.1042/BSR20160195. Epub 2016 Aug 18. Biosci Rep. 2016. PMID: 27538678 Free PMC article.
-
Role of apolipoprotein E in renal damage protection.Histochem Cell Biol. 2011 Jun;135(6):571-9. doi: 10.1007/s00418-011-0815-1. Epub 2011 May 15. Histochem Cell Biol. 2011. PMID: 21573735
-
The Roles of Fatty Acids and Apolipoproteins in the Kidneys.Metabolites. 2022 May 20;12(5):462. doi: 10.3390/metabo12050462. Metabolites. 2022. PMID: 35629966 Free PMC article. Review.
-
Entacapone alleviates muscle atrophy by modulating oxidative stress, proteolysis, and lipid aggregation in multiple mice models.Front Physiol. 2024 Dec 9;15:1483594. doi: 10.3389/fphys.2024.1483594. eCollection 2024. Front Physiol. 2024. PMID: 39717825 Free PMC article.
-
Recent progress in histochemistry and cell biology.Histochem Cell Biol. 2012 Apr;137(4):403-57. doi: 10.1007/s00418-012-0933-4. Epub 2012 Feb 25. Histochem Cell Biol. 2012. PMID: 22366957 Review.
References
-
- Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms and potential consequences. Am J Physiol, Renal Physiol. 2006;290:262–272. - PubMed
-
- Attman PO, Alaupovic P, Samuelsson O. Lipoprotein abnormalities as a risk factor for progressive nondiabetic renal disease. Kidney Int Suppl. 1999;71:14–17. - PubMed
-
- Cases A, Coll E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl. 2005;99:87–93. - PubMed
-
- Dalrymple LS, Kaysen GA. The effect of lipoproteins on the development and progression of renal disease. Am J Nephrol. 2008;28:723–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

