The maize mixed-linkage (1->3),(1->4)-beta-D-glucan polysaccharide is synthesized at the golgi membrane
- PMID: 20488897
- PMCID: PMC2899932
- DOI: 10.1104/pp.110.156158
The maize mixed-linkage (1->3),(1->4)-beta-D-glucan polysaccharide is synthesized at the golgi membrane
Abstract
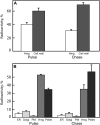
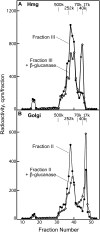
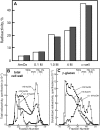
With the exception of cellulose and callose, the cell wall polysaccharides are synthesized in Golgi membranes, packaged into vesicles, and exported to the plasma membrane where they are integrated into the microfibrillar structure. Consistent with this paradigm, several published reports have shown that the maize (Zea mays) mixed-linkage (1-->3),(1-->4)-beta-D-glucan, a polysaccharide that among angiosperms is unique to the grasses and related Poales species, is synthesized in vitro with isolated maize coleoptile Golgi membranes and the nucleotide-sugar substrate, UDP-glucose. However, a recent study reported the inability to detect the beta-glucan immunocytochemically at the Golgi, resulting in a hypothesis that the mixed-linkage beta-glucan oligomers may be initiated at the Golgi but are polymerized at the plasma membrane surface. Here, we demonstrate that (1-->3),(1-->4)-beta-D-glucans are detected immunocytochemically at the Golgi of the developing maize coleoptiles. Further, when maize seedlings at the third-leaf stage were pulse labeled with [(14)C]O(2) and Golgi membranes were isolated from elongating cells at the base of the developing leaves, (1-->3),(1-->4)-beta-D-glucans of an average molecular mass of 250 kD and higher were detected in isolated Golgi membranes. When the pulse was followed by a chase period, the labeled polysaccharides of the Golgi membrane diminished with subsequent transfer to the cell wall. (1-->3),(1-->4)-beta-D-Glucans of at least 250 kD were isolated from cell walls, but much larger aggregates were also detected, indicating a potential for intermolecular interactions with glucuronoarabinoxylans or intermolecular grafting in muro.
Figures



Similar articles
-
Determining the subcellular location of synthesis and assembly of the cell wall polysaccharide (1,3; 1,4)-β-D-glucan in grasses.Plant Cell. 2015 Mar;27(3):754-71. doi: 10.1105/tpc.114.135970. Epub 2015 Mar 13. Plant Cell. 2015. PMID: 25770111 Free PMC article.
-
Synthesis of (1-->3), (1-->4)-beta-D-glucan in the Golgi apparatus of maize coleoptiles.Proc Natl Acad Sci U S A. 1993 May 1;90(9):3850-4. doi: 10.1073/pnas.90.9.3850. Proc Natl Acad Sci U S A. 1993. PMID: 8483902 Free PMC article.
-
In the grass species Brachypodium distachyon, the production of mixed-linkage (1,3;1,4)-β-glucan (MLG) occurs in the Golgi apparatus.Plant J. 2018 Mar;93(6):1062-1075. doi: 10.1111/tpj.13830. Epub 2018 Mar 6. Plant J. 2018. PMID: 29377449
-
(1,3;1,4)-beta-D-glucans in cell walls of the poaceae, lower plants, and fungi: a tale of two linkages.Mol Plant. 2009 Sep;2(5):873-82. doi: 10.1093/mp/ssp063. Epub 2009 Aug 17. Mol Plant. 2009. PMID: 19825664 Review.
-
Cellulose and callose synthesis and organization in focus, what's new?Curr Opin Plant Biol. 2016 Dec;34:9-16. doi: 10.1016/j.pbi.2016.07.007. Epub 2016 Jul 29. Curr Opin Plant Biol. 2016. PMID: 27479608 Review.
Cited by
-
Critical Review of Plant Cell Wall Matrix Polysaccharide Glycosyltransferase Activities Verified by Heterologous Protein Expression.Front Plant Sci. 2019 Jul 16;10:915. doi: 10.3389/fpls.2019.00915. eCollection 2019. Front Plant Sci. 2019. PMID: 31379900 Free PMC article. Review.
-
Cell type specific transcriptional reprogramming of maize leaves during Ustilago maydis induced tumor formation.Sci Rep. 2019 Jul 15;9(1):10227. doi: 10.1038/s41598-019-46734-3. Sci Rep. 2019. PMID: 31308451 Free PMC article.
-
Genome wide comprehensive analysis and web resource development on cell wall degrading enzymes from phyto-parasitic nematodes.BMC Plant Biol. 2015 Aug 1;15:187. doi: 10.1186/s12870-015-0576-4. BMC Plant Biol. 2015. PMID: 26232118 Free PMC article.
-
Hemicellulose biosynthesis.Planta. 2013 Oct;238(4):627-42. doi: 10.1007/s00425-013-1921-1. Epub 2013 Jun 26. Planta. 2013. PMID: 23801299 Review.
-
Enzymes in 3D: Synthesis, remodelling, and hydrolysis of cell wall (1,3;1,4)-β-glucans.Plant Physiol. 2023 Dec 30;194(1):33-50. doi: 10.1093/plphys/kiad415. Plant Physiol. 2023. PMID: 37594400 Free PMC article.
References
-
- Anderson MA, Stone BA. (1975) A new substrate for investigating the specificity of β-glucan hydrolases. FEBS Lett 52: 202–207 - PubMed
-
- Buckeridge MS, Vergara CE, Carpita NC. (2001) Insight into multi-site mechanisms of glycosyl transfer in (1→4)-β-D-glycan synthases provided by the cereal mixed-linkage (1→3),(1→4)-β-D-glucan synthase. Phytochemistry 57: 1045–1053 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

