Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells
- PMID: 20488942
- PMCID: PMC2905406
- DOI: 10.1093/toxsci/kfq148
Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells
Abstract
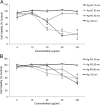
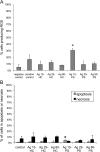
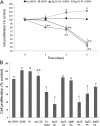
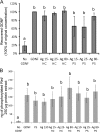
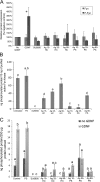
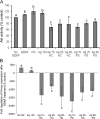
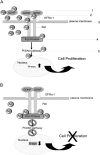
Silver nanoparticles (Ag-NPs) are being utilized in an increasing number of fields and are components of antibacterial coatings, antistatic materials, superconductors, and biosensors. A number of reports have now described the toxic effects of silver nanoparticles on somatic cells; however, no study has examined their effects on the germ line at the molecular level. Spermatogenesis is a complex biological process that is particularly sensitive to environmental insults. Many chemicals, including ultrafine particles, have a negative effect on the germ line, either by directly affecting the germ cells or by indirectly acting on the somatic cells of the testis. In the present study, we have assessed the impact of different doses of Ag-NPs, as well as their size and biocompatible coating, on the proliferation of mouse spermatogonial stem cells (SSCs), which are at the origin of the germ line in the adult testis. At concentrations >OR= 10 microg/ml, Ag-NPs induced a significant decline in SSCs proliferation, which was also dependent on their size and coating. At the concentration of 10 microg/ml, reactive oxygen species production and/or apoptosis did not seem to play a major role; therefore, we explored other mechanisms to explain the decrease in cell proliferation. Because glial cell line-derived neurotrophic factor (GDNF) is vital for SSC self-renewal in vitro and in vivo, we evaluated the effects of Ag-NPs on GDNF-mediated signaling in these cells. Although the nanoparticles did not reduce GDNF binding or Ret receptor activity, our data revealed that already at a concentration of 10 microg/ml, silver nanoparticles specifically interact with Fyn kinase downstream of Ret and impair SSC proliferation in vitro. In addition, we demonstrated that the particle coating was degraded upon interaction with the intracellular microenvironment, reducing biocompatibility.
Figures



Similar articles
-
GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells.Genes Cells. 2008 Apr;13(4):365-74. doi: 10.1111/j.1365-2443.2008.01171.x. Genes Cells. 2008. PMID: 18363967
-
Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal.Stem Cell Reports. 2015 Mar 10;4(3):489-502. doi: 10.1016/j.stemcr.2015.01.010. Epub 2015 Feb 12. Stem Cell Reports. 2015. PMID: 25684228 Free PMC article.
-
Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells.Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16489-94. doi: 10.1073/pnas.0407063101. Epub 2004 Nov 1. Proc Natl Acad Sci U S A. 2004. PMID: 15520394 Free PMC article.
-
[Regulatory effect of GDNF on the proliferation and differentiation of mammalian spermatogonial stem cells].Zhonghua Nan Ke Xue. 2011 Jul;17(7):628-33. Zhonghua Nan Ke Xue. 2011. PMID: 21823348 Review. Chinese.
-
Regulation of spermatogonial stem cell self-renewal in mammals.Annu Rev Cell Dev Biol. 2008;24:263-86. doi: 10.1146/annurev.cellbio.24.110707.175355. Annu Rev Cell Dev Biol. 2008. PMID: 18588486 Free PMC article. Review.
Cited by
-
Evaluating the effect of silver nanoparticles on testes of adult albino rats (histological, immunohistochemical and biochemical study).J Mol Histol. 2017 Feb;48(1):9-27. doi: 10.1007/s10735-016-9701-4. Epub 2016 Nov 1. J Mol Histol. 2017. PMID: 27803997
-
Putative adverse outcome pathways for silver nanoparticle toxicity on mammalian male reproductive system: a literature review.Part Fibre Toxicol. 2023 Jan 5;20(1):1. doi: 10.1186/s12989-022-00511-9. Part Fibre Toxicol. 2023. PMID: 36604752 Free PMC article.
-
Effect of beta-carotene on titanium oxide nanoparticles-induced testicular toxicity in mice.J Assist Reprod Genet. 2014 May;31(5):561-8. doi: 10.1007/s10815-014-0184-5. Epub 2014 Feb 11. J Assist Reprod Genet. 2014. PMID: 24515782 Free PMC article.
-
Engineered nanomaterials: an emerging class of novel endocrine disruptors.Biol Reprod. 2014 Jul;91(1):20. doi: 10.1095/biolreprod.113.116244. Epub 2014 Jun 4. Biol Reprod. 2014. PMID: 24899576 Free PMC article. Review.
-
Biosilver nanoparticle interface offers improved cell viability.Surf Innov. 2016 Sep;4(3):121-132. doi: 10.1680/jsuin.16.00010. Epub 2016 Nov 7. Surf Innov. 2016. PMID: 29057075 Free PMC article.
References
-
- Ahamed M, Karns M, Goodson M, Rowe J, Hussain S, Schlager J, Hong Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008;233:404–410. - PubMed
-
- Ahamed M, Posgai R, Gorey T, Nielsen M, Hussain S, Rowe J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. 2010;242:263–269. - PubMed
-
- Asharani P, Low Kah Mun G, Hande M, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

