Genetic loci modulate macrophage activity and glomerular damage in experimental glomerulonephritis
- PMID: 20488952
- PMCID: PMC3152224
- DOI: 10.1681/ASN.2009090968
Genetic loci modulate macrophage activity and glomerular damage in experimental glomerulonephritis
Abstract
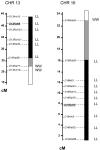
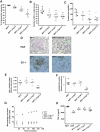
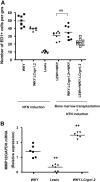
The Wistar Kyoto (WKY) rat is uniquely susceptible to experimentally induced crescentic glomerulonephritis. Two major quantitative trait loci (QTLs) on chromosomes 13 (Crgn1) and 16 (Crgn2) with logarithm of odds >8, as well as five other loci (Crgn3 through 7), largely explain this genetic susceptibility. To understand further the effects of Crgn1 and Crgn2, we generated a double-congenic strain by introgressing these loci from glomerulonephritis-resistant Lewis rats onto the WKY genetic background. Induction of nephrotoxic nephritis in the double-congenic rats (WKY.LCrgn1,2) produced markedly fewer glomerular crescents, reduced macrophage infiltration, and decreased expression of glomerular TNF-alpha and inducible nitric oxide synthase expression compared with control animals. Bone marrow and kidney transplantation studies between parental and WKY.LCrgn1,2 strains, together with in vitro experiments, demonstrated that Crgn1 and Crgn2 contribute exclusively to circulating cell-related glomerular injury by regulating macrophage infiltration and activation. The residual genetic susceptibility to crescentic glomerulonephritis in WKY.LCrgn1,2 rats associated with macrophage activity (especially with enhanced metalloelastase expression) rather than macrophage infiltration. Taken together, these results demonstrate that a genetic influence on macrophage activation, rather than number, determines glomerular damage in immune-mediated glomerulonephritis.
Figures

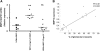
References
-
- Jennette JC, Heptinstall RH, Ovid Technologies Inc. : Heptinstall's Pathology of the Kidney, 6th Ed., Philadelphia, Lippincott Williams & Wilkins, 2007
-
- Tipping PG: Crescentic nephritis: Is it in your genes? Nephrol Dial Transplant 23: 3065–3066, 2008 - PubMed
-
- Tam FW, Smith J, Morel D, Karkar AM, Thompson EM, Cook HT, Pusey CD: Development of scarring and renal failure in a rat model of crescentic glomerulonephritis. Nephrol Dial Transplant 14: 1658–1666, 1999 - PubMed
-
- Smith J, Lai PC, Behmoaras J, Roufosse C, Bhangal G, McDaid JP, Aitman T, Tam FW, Pusey CD, Cook HT: Genes expressed by both mesangial cells and bone marrow-derived cells underlie genetic susceptibility to crescentic glomerulonephritis in the rat. J Am Soc Nephrol 18: 1816–1823, 2007 - PubMed
-
- Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT: Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439: 851–855, 2006 - PubMed

