Myometrial wound healing post-Cesarean delivery in the MRL/MpJ mouse model of uterine scarring
- PMID: 20489145
- PMCID: PMC2893663
- DOI: 10.2353/ajpath.2010.091209
Myometrial wound healing post-Cesarean delivery in the MRL/MpJ mouse model of uterine scarring
Abstract
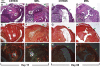
There is little known about healing of the uterus after Cesarean delivery (CD). Uterine wound repair was studied by using two strains of mice with different wound healing characteristics: MRL/MpJ(+/+) (MRL: "high-healer" phenotype) and C57Bl/6 ("low-healer" phenotype). First, we examined the morphology and histology of the uterine wall repair. We identified wound granulation tissue 3 days post-CD in both strains, albeit less in the MRL strain. Macroscopically, no scar could be identified either in MRL or C57Bl/6 mice on day 60 post-CD. However, histologically, we found significant differences in wound integration, inflammation, and collagen birefringence between the two strains of mice. Using a histological index, we provided evidence for significant differences in mitotic activity in the initial phases of uterine healing among strains. Functional behavior of the uterine scar also was analyzed by using biomechanical parameters such as slope (measure of stiffness), yield point (measure of elasticity), and break point (measure of strength). There were significant differences in stiffness of the scarred myometrium between the two phenotypes. MRL mice displayed a significantly lower yield point compared with C57Bl/6. The break point was reached faster on days 15 and 60 in both C57Bl/6 and MRL strains compared with day 3 post-CD. Our findings indicate that differences in regenerative ability translate in histological, mitotic, and functional differences in biomechanical properties of the scarred myometrium after CD.
Figures




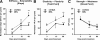
Similar articles
-
The effect of dystocia and previous cesarean uterine scar on the tensile properties of the lower uterine segment.Am J Obstet Gynecol. 2006 Mar;194(3):873-83. doi: 10.1016/j.ajog.2005.09.004. Am J Obstet Gynecol. 2006. PMID: 16522428
-
Uncorrelated healing response of tendon and ear injuries in MRL highlight a role for the local tendon environment in driving scarless healing.Connect Tissue Res. 2018 Sep;59(5):472-482. doi: 10.1080/03008207.2018.1485665. Epub 2018 Jun 21. Connect Tissue Res. 2018. PMID: 29929396 Free PMC article.
-
Skin wounds in the MRL/MPJ mouse heal with scar.Wound Repair Regen. 2006 Jan-Feb;14(1):81-90. doi: 10.1111/j.1524-475X.2005.00092.x. Wound Repair Regen. 2006. PMID: 16476076
-
"Niche" czyli ubytek w miejscu blizny mięśniówki macicy po cięciu cesarskim - przyczyny, diagnostyka, objawy.Ginekol Pol. 2016;87(2):143-7. doi: 10.17772/gp/60072. Ginekol Pol. 2016. PMID: 27306292 Review. English, Polish.
-
Regeneration of articular cartilage in healer and non-healer mice.Matrix Biol. 2014 Oct;39:50-5. doi: 10.1016/j.matbio.2014.08.011. Epub 2014 Aug 28. Matrix Biol. 2014. PMID: 25173437 Free PMC article. Review.
Cited by
-
Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a retrospective cohort study.BMC Womens Health. 2014 Sep 24;14:116. doi: 10.1186/1472-6874-14-116. BMC Womens Health. 2014. PMID: 25248928 Free PMC article.
-
Inflammation and Its Correlates in Regenerative Wound Healing: An Alternate Perspective.Adv Wound Care (New Rochelle). 2014 Sep 1;3(9):592-603. doi: 10.1089/wound.2014.0528. Adv Wound Care (New Rochelle). 2014. PMID: 25207202 Free PMC article. Review.
-
Rupture of the posterior cul-de-sac during trial of labour after caesarean section.BMJ Case Rep. 2017 Dec 2;2017:bcr2017221149. doi: 10.1136/bcr-2017-221149. BMJ Case Rep. 2017. PMID: 29197839 Free PMC article.
-
Exploring the Effects of Standardized Soft Tissue Mobilization on the Viscoelastic Properties, Pressure Pain Thresholds, and Tactile Pressure Thresholds of the Cesarean Section Scar.J Integr Complement Med. 2022 Apr;28(4):355-362. doi: 10.1089/jicm.2021.0178. Epub 2022 Jan 13. J Integr Complement Med. 2022. PMID: 35426735 Free PMC article. Clinical Trial.
-
Full-length human placental sFlt-1-e15a isoform induces distinct maternal phenotypes of preeclampsia in mice.PLoS One. 2015 Apr 10;10(4):e0119547. doi: 10.1371/journal.pone.0119547. eCollection 2015. PLoS One. 2015. PMID: 25860260 Free PMC article.
References
-
- Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2007. Natl Vital Stat Rep. 2009;57:1–23. - PubMed
-
- Williams JW. A critical analysis of 21 years experience with cesarean section. Bull Johns Hopkins Hosp. 1921;32:173–190.
-
- Repina MM. Case reports on uterine rupture in pregnancy after cesarean section. Akush Ginekol (Mosk) 1955;6:61–62. - PubMed
-
- Goss RJ, Grimes LN. Epidermal downgrowths in regenerating rabbit ear holes. J Morphol. 1975;146:533–542. - PubMed
-
- Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

