Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex
- PMID: 20489158
- PMCID: PMC2893675
- DOI: 10.2353/ajpath.2010.090937
Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex
Abstract
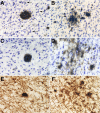
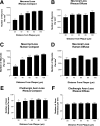
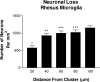
The amyloid-beta peptide (Abeta) deposited in plaques in Alzheimer's disease has been shown to cause degeneration of neurons in experimental paradigms in vivo and in vitro. However, it has been difficult to convincingly demonstrate toxicity of native amyloid deposits in the aged and Alzheimer brains. Here we provide evidence that the fibrillar conformation of Abeta (fAbeta) deposited in compact plaques is associated with the pathologies observed in Alzheimer brains. fAbeta containing compact but not diffuse plaques in the aged rhesus cortex contained activated microglia and clusters of phosphorylated tau-positive swollen neurites. Scholl's quantitative analysis revealed that the area adjacent to fAbeta, containing compact but not diffuse plaques in aged rhesus, aged human, and Alzheimer's disease cortex, displays significant loss of neurons and small but statistically significant reduction in the density of cholinergic axons. These observations suggest that fAbeta toxicity may not be restricted to cultured cells and animal injection models. Rather, fAbeta deposited in native compact plaques in aged and AD brains may exert selective toxic effects on its surrounding neural environment.
Figures


Similar articles
-
Trem2 Deletion Reduces Late-Stage Amyloid Plaque Accumulation, Elevates the Aβ42:Aβ40 Ratio, and Exacerbates Axonal Dystrophy and Dendritic Spine Loss in the PS2APP Alzheimer's Mouse Model.J Neurosci. 2020 Feb 26;40(9):1956-1974. doi: 10.1523/JNEUROSCI.1871-19.2019. Epub 2020 Jan 24. J Neurosci. 2020. PMID: 31980586 Free PMC article.
-
Microglia activation mediates fibrillar amyloid-β toxicity in the aged primate cortex.Neurobiol Aging. 2011 Mar;32(3):387-97. doi: 10.1016/j.neurobiolaging.2009.02.025. Epub 2009 Apr 5. Neurobiol Aging. 2011. PMID: 19349094 Free PMC article.
-
The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer's disease.Neuroscience. 2001;105(1):99-107. doi: 10.1016/s0306-4522(01)00169-5. Neuroscience. 2001. PMID: 11483304
-
Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease.J Cell Mol Med. 2008 Oct;12(5B):1848-62. doi: 10.1111/j.1582-4934.2008.00411.x. Epub 2008 Jul 10. J Cell Mol Med. 2008. PMID: 18624777 Free PMC article. Review.
-
Does beta-amyloid plaque formation cause structural injury to neuronal processes?Neurotox Res. 2005;7(1-2):5-15. doi: 10.1007/BF03033772. Neurotox Res. 2005. PMID: 15639794 Review.
Cited by
-
An overview on microglial origin, distribution, and phenotype in Alzheimer's disease.J Cell Physiol. 2024 Jun;239(6):e30829. doi: 10.1002/jcp.30829. Epub 2022 Jul 13. J Cell Physiol. 2024. PMID: 35822939 Free PMC article. Review.
-
Regional and Gender Study of Neuronal Density in Brain during Aging and in Alzheimer's Disease.Front Aging Neurosci. 2016 Sep 13;8:213. doi: 10.3389/fnagi.2016.00213. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27679571 Free PMC article.
-
Fingolimod modulates multiple neuroinflammatory markers in a mouse model of Alzheimer's disease.Sci Rep. 2016 Apr 27;6:24939. doi: 10.1038/srep24939. Sci Rep. 2016. PMID: 27117087 Free PMC article.
-
Calorie restriction attenuates astrogliosis but not amyloid plaque load in aged rhesus macaques: a preliminary quantitative imaging study.Brain Res. 2013 May 1;1508:1-8. doi: 10.1016/j.brainres.2013.02.046. Epub 2013 Mar 7. Brain Res. 2013. PMID: 23473840 Free PMC article.
-
Immunotherapy for the treatment of Alzheimer's disease: amyloid-β or tau, which is the right target?Immunotargets Ther. 2013 Dec 27;3:19-28. doi: 10.2147/ITT.S40131. eCollection 2014. Immunotargets Ther. 2013. PMID: 27471697 Free PMC article. Review.
References
-
- Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann NY Acad Sci. 2000;924:17–25. - PubMed
-
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563:311–314. - PubMed
-
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochem. 2000;39:18031–18039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

