Ligand regulation of the quaternary organization of cell surface M3 muscarinic acetylcholine receptors analyzed by fluorescence resonance energy transfer (FRET) imaging and homogeneous time-resolved FRET
- PMID: 20489201
- PMCID: PMC2906324
- DOI: 10.1074/jbc.M110.122184
Ligand regulation of the quaternary organization of cell surface M3 muscarinic acetylcholine receptors analyzed by fluorescence resonance energy transfer (FRET) imaging and homogeneous time-resolved FRET
Abstract
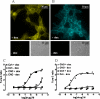
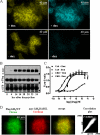
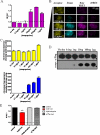
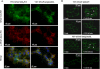
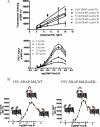
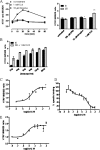
Flp-In(TM) T-REx(TM) 293 cells expressing a wild type human M(3) muscarinic acetylcholine receptor construct constitutively and able to express a receptor activated solely by synthetic ligand (RASSL) form of this receptor on demand maintained response to the muscarinic agonist carbachol but developed response to clozapine N-oxide only upon induction of the RASSL. The two constructs co-localized at the plasma membrane and generated strong ratiometric fluorescence resonance energy transfer (FRET) signals consistent with direct physical interactions. Increasing levels of induction of the FRET donor RASSL did not alter wild type receptor FRET-acceptor levels substantially. However, ratiometric FRET was modulated in a bell-shaped fashion with maximal levels of the donor resulting in decreased FRET. Carbachol, but not the antagonist atropine, significantly reduced the FRET signal. Cell surface homogeneous time-resolved FRET, based on SNAP-tag technology and employing wild type and RASSL forms of the human M(3) receptor expressed stably in Flp-In(TM) TREx(TM) 293 cells, also identified cell surface dimeric/oligomeric complexes. Now, however, signals were enhanced by appropriate selective agonists. At the wild type receptor, large increases in FRET signal to carbachol and acetylcholine were concentration-dependent with EC(50) values consistent with the relative affinities of the two ligands. These studies confirm the capacity of the human M(3) muscarinic acetylcholine receptor to exist as dimeric/oligomeric complexes at the surface of cells and demonstrate that the organization of such complexes can be modified by ligand binding. However, conclusions as to the effect of ligands on such complexes may depend on the approach used.
Figures
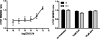
References
-
- Milligan G. (2004) Mol. Pharmacol. 66, 1–7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

