The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane
- PMID: 20489726
- PMCID: PMC2952662
- DOI: 10.1038/cdd.2010.63
The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane
Abstract
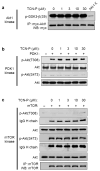
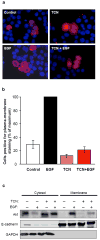
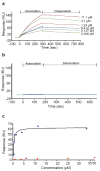
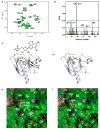
Persistently hyperphosphorylated Akt contributes to human oncogenesis and resistance to therapy. Triciribine (TCN) phosphate (TCN-P), the active metabolite of the Akt phosphorylation inhibitor TCN, is in clinical trials, but the mechanism by which TCN-P inhibits Akt phosphorylation is unknown. Here we show that in vitro, TCN-P inhibits neither Akt activity nor the phosphorylation of Akt S473 and T308 by mammalian target of rapamycin or phosphoinositide-dependent kinase 1. However, in intact cells, TCN inhibits EGF-stimulated Akt recruitment to the plasma membrane and phosphorylation of Akt. Surface plasmon resonance shows that TCN, but not TCN, binds Akt-derived pleckstrin homology (PH) domain (K(D): 690 nM). Furthermore, nuclear magnetic resonance spectroscopy shows that TCN-P, but not TCN, binds to the PH domain in the vicinity of the PIP3-binding pocket. Finally, constitutively active Akt mutants, Akt1-T308D/S473D and myr-Akt1, but not the transforming mutant Akt1-E17K, are resistant to TCN and rescue from its inhibition of proliferation and induction of apoptosis. Thus, the results of our studies indicate that TCN-P binds to the PH domain of Akt and blocks its recruitment to the membrane, and that the subsequent inhibition of Akt phosphorylation contributes to TCN-P antiproliferative and proapoptotic activities, suggesting that this drug may be beneficial to patients whose tumors express persistently phosphorylated Akt.
Conflict of interest statement
Figures
Similar articles
-
Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT.Invest New Drugs. 2011 Dec;29(6):1381-9. doi: 10.1007/s10637-010-9479-2. Epub 2010 Jul 20. Invest New Drugs. 2011. PMID: 20644979 Free PMC article. Clinical Trial.
-
A small molecule inhibits Akt through direct binding to Akt and preventing Akt membrane translocation.J Biol Chem. 2010 Mar 12;285(11):8383-94. doi: 10.1074/jbc.M109.094060. Epub 2010 Jan 12. J Biol Chem. 2010. Retraction in: J Biol Chem. 2016 Oct 21;291(43):22856. doi: 10.1074/jbc.A109.094060. PMID: 20068047 Free PMC article. Retracted.
-
Inhibition of Akt kinase activity by a peptide spanning the betaA strand of the proto-oncogene TCL1.J Biol Chem. 2004 Dec 17;279(51):53407-18. doi: 10.1074/jbc.M403775200. Epub 2004 Sep 30. J Biol Chem. 2004. PMID: 15459205
-
NFluc-FHA2-Aktpep-CFluc.2008 Nov 14 [updated 2008 Dec 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Nov 14 [updated 2008 Dec 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641681 Free Books & Documents. Review.
-
Phosphorylation of Akt at the C-terminal tail triggers Akt activation.Cell Cycle. 2014;13(14):2162-4. doi: 10.4161/cc.29584. Epub 2014 Jun 16. Cell Cycle. 2014. PMID: 24933731 Free PMC article. Review.
Cited by
-
Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque.PLoS One. 2014 Mar 5;9(3):e90563. doi: 10.1371/journal.pone.0090563. eCollection 2014. PLoS One. 2014. PMID: 24599185 Free PMC article.
-
New drug-like small molecule antagonizes phosphatidylinositol (3,4,5)-trisphosphate (PIP3) in patients with conotruncal heart defects.J Taibah Univ Med Sci. 2023 May 4;18(6):1244-1253. doi: 10.1016/j.jtumed.2023.04.006. eCollection 2023 Dec. J Taibah Univ Med Sci. 2023. PMID: 37250809 Free PMC article.
-
The Role of ZNF275/AKT Pathway in Carcinogenesis and Cisplatin Chemosensitivity of Cervical Cancer Using Patient-Derived Xenograft Models.Cancers (Basel). 2023 Nov 28;15(23):5625. doi: 10.3390/cancers15235625. Cancers (Basel). 2023. PMID: 38067329 Free PMC article.
-
Akt2 and acid ceramidase cooperate to induce cell invasion and resistance to apoptosis.Cell Cycle. 2013 Jul 1;12(13):2024-32. doi: 10.4161/cc.25043. Epub 2013 Jun 6. Cell Cycle. 2013. PMID: 23777806 Free PMC article.
-
The Biological Role of Nestin(+)-Cells in Physiological and Pathological Cardiovascular Remodeling.Front Cell Dev Biol. 2018 Feb 14;6:15. doi: 10.3389/fcell.2018.00015. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 29492403 Free PMC article. Review.
References
-
- Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. - PubMed
-
- Bellacosa A, Testa HR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. - PubMed
-
- Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2008;7:2991–2996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

