Autocrine, not paracrine, interferon-gamma gene delivery enhances ex vivo antigen-specific cytotoxic T lymphocyte stimulation and killing
- PMID: 20490265
- PMCID: PMC2871187
- DOI: 10.1155/2010/270985
Autocrine, not paracrine, interferon-gamma gene delivery enhances ex vivo antigen-specific cytotoxic T lymphocyte stimulation and killing
Abstract
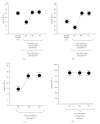
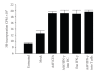
The adoptive transfer of antigen-specific cytotoxic T lymphocytes (CTL) shows promise in the treatment of cancer and infectious diseases. We utilize adeno-associated virus-(AAV-) based antigen gene-loaded dendritic cells (DCs) to stimulate such antigen-specific CTL. Yet further improvements in CTL stimulation and killing may result by gene delivery of various Th1-response interferons/cytokines, such as interferon gamma (IFN-gamma), as the delivered gene can continuously produce that interferon. However which immune cell type should optimally express IFN-gamma is unclear as the phenotypes of both DC and T cells are enhanced by it. Here, we used AAV to compare and contrast IFN-gamma gene delivery into DC or T cells, and versus the addition of exogenous IFN-gamma, for stimulating carcinoembryonic antigen-(CEA-) specific CTL. It was found that AAV/IFN-gamma delivery into T cells (autocrine) resulted in T cell populations with the highest CD8(+)/CD4(+) ratio, highest IFN-gamma(+)/IL-4(+) ratio, highest CD69(+),CD8(+) levels, and lowest CD4(+)/CD25(+) levels, all consistent with the strongest Th1 response. Most importantly, AAV/IFN-gamma transduction of T cells resulted in antigen-specific T cell populations with the highest killing capabilities, 49% above other treatments. These data strongly suggest that AAV/IFN-gamma autocrine gene delivery into T cells is worthy of further study towards maximizing the generation of antigen-specific anticancer CTL killers.
Figures

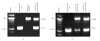



Similar articles
-
Comparison of AAV/IL-7 autocrine (T cell) versus paracrine (DC) gene delivery for enhancing CTL stimulation and function.Cancer Immunol Immunother. 2010 May;59(5):779-87. doi: 10.1007/s00262-009-0798-0. Epub 2009 Nov 29. Cancer Immunol Immunother. 2010. PMID: 20091029 Free PMC article.
-
HBV genes induce cytotoxic T-lymphocyte response upon adeno-associated virus (AAV) vector delivery into dendritic cells.J Viral Hepat. 2006 Sep;13(9):605-12. doi: 10.1111/j.1365-2893.2006.00734.x. J Viral Hepat. 2006. PMID: 16907847
-
[Dendritic cells infected by recombinant adeno-associated virus with CEA gene to induce antigen-specific CTL response to CD44(+)CD24(-/low) cells from MCF-7 breast cancer cell line].Beijing Da Xue Xue Bao Yi Xue Ban. 2011 Apr 18;43(2):173-8. Beijing Da Xue Xue Bao Yi Xue Ban. 2011. PMID: 21503107 Chinese.
-
Host-oriented peptide evaluation using whole blood assay for generating antigen-specific cytotoxic T lymphocytes.Anticancer Res. 2004 Mar-Apr;24(2C):1193-200. Anticancer Res. 2004. PMID: 15154646
-
[Towards novel tuberculosis and leprosy vaccine development: the role of Th1-inducing peptide in cytotoxic T cell differentiation].Nihon Hansenbyo Gakkai Zasshi. 2013 Dec;82(3):111-7. doi: 10.5025/hansen.82.111. Nihon Hansenbyo Gakkai Zasshi. 2013. PMID: 24579458 Review. Japanese.
Cited by
-
AAV2/IL-12 gene delivery into dendritic cells (DC) enhances CTL stimulation above other IL-12 applications: Evidence for IL-12 intracrine activity in DC.Oncoimmunology. 2012 Sep 1;1(6):847-855. doi: 10.4161/onci.20504. Oncoimmunology. 2012. PMID: 23162752 Free PMC article.
References
-
- Park JR, DiGiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Molecular Therapy. 2007;15(4):825–833. - PubMed
-
- Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. Journal of Clinical Oncology. 2006;24(31):5060–5069. - PubMed
-
- Santin AD, Hermonat PL, Ravaggi A, et al. Development and therapeutic effect of adoptively transferred T cells primed by tumor lysate-pulsed autologous dendritic cells in a patient with metastatic endometrial cancer. Gynecologic and Obstetric Investigation. 2000;49(3):194–203. - PubMed
-
- Santin AD, Hermonat PL, Ravaggi A, et al. Development, characterization and distribution of adoptively transferred peripheral blood lymphocytes primed by human papillomavirus 18 E7-pulsed autologous dendritic cells in a patient with metastatic adenocarcinoma of the uterine cervix. European Journal of Gynaecological Oncology. 2000;21(1):17–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

