β-Glucosidases
- PMID: 20490603
- PMCID: PMC11115901
- DOI: 10.1007/s00018-010-0399-2
β-Glucosidases
Abstract
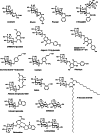
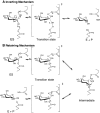
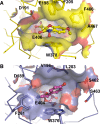
β-Glucosidases (3.2.1.21) are found in all domains of living organisms, where they play essential roles in the removal of nonreducing terminal glucosyl residues from saccharides and glycosides. β-Glucosidases function in glycolipid and exogenous glycoside metabolism in animals, defense, cell wall lignification, cell wall β-glucan turnover, phytohormone activation, and release of aromatic compounds in plants, and biomass conversion in microorganisms. These functions lead to many agricultural and industrial applications. β-Glucosidases have been classified into glycoside hydrolase (GH) families GH1, GH3, GH5, GH9, and GH30, based on their amino acid sequences, while other β-glucosidases remain to be classified. The GH1, GH5, and GH30 β-glucosidases fall in GH Clan A, which consists of proteins with (β/α)(8)-barrel structures. In contrast, the active site of GH3 enzymes comprises two domains, while GH9 enzymes have (α/α)(6) barrel structures. The mechanism by which GH1 enzymes recognize and hydrolyze substrates with different specificities remains an area of intense study.
Figures
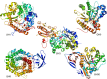
References
-
- Opassiri R, Pomthong B, Akiyama T, Nakphaichit M, Onkoksoong T, Ketudat-Cairns M, Ketudat Cairns JR. A stress-induced rice β-glucosidase represents a new subfamily of glycosyl hydrolase family 5 containing a fascin-like domain. Biochem J. 2007;408:241–249. doi: 10.1042/BJ20070734. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

