Community structure, trophic position and reproductive mode of soil and bark-living oribatid mites in an alpine grassland ecosystem
- PMID: 20490626
- PMCID: PMC2951506
- DOI: 10.1007/s10493-010-9366-8
Community structure, trophic position and reproductive mode of soil and bark-living oribatid mites in an alpine grassland ecosystem
Abstract
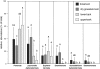
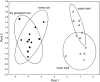
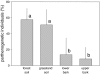
The community structure, stable isotope ratios ((15)N/(14)N, (13)C/(12)C) and reproductive mode of oribatid mites (Acari, Oribatida) were investigated in four habitats (upper tree bark, lower tree bark, dry grassland soil, forest soil) at two sites in the Central Alps (Tyrol, Austria). We hypothesized that community structure and trophic position of oribatid mites of dry grassland soils and bark of trees are similar since these habitats have similar abiotic characteristics (open, dry) compared with forest soil. Further, we hypothesized that derived taxa of oribatid mites reproducing sexually dominate on the bark of trees since species in this habitat consume living resources such as lichens. In contrast to our hypothesis, the community structure of oribatid mites differed among grassland, forest and bark indicating the existence of niche differentiation in the respective oribatid mite species. In agreement with our hypothesis, sexually reproducing taxa of oribatid mites dominated on the bark of trees whereas parthenogenetic species were more frequent in soil. Several species of bark-living oribatid mites had stable isotope signatures that were similar to lichens indicating that they feed on lichens. However, nine species that frequently occurred on tree bark did not feed on lichens according to their stable isotope signatures. No oribatid mite species could be ascribed to moss feeding. We conclude that sexual reproduction served as preadaptation for oribatid mites allowing them to exploit new habitats and new resources on the bark of trees. Abiotic factors likely are of limited importance for bark-living oribatid mites since harsh abiotic conditions are assumed to favor parthenogenesis.
Figures



Similar articles
-
Positive correlation of trophic level and proportion of sexual taxa of oribatid mites (Acari: Oribatida) in alpine soil systems.Exp Appl Acarol. 2014 Aug;63(4):465-79. doi: 10.1007/s10493-014-9801-3. Epub 2014 Apr 1. Exp Appl Acarol. 2014. PMID: 24687174
-
The trophic structure of bark-living oribatid mite communities analysed with stable isotopes ((15)N, (13)C) indicates strong niche differentiation.Exp Appl Acarol. 2007;41(1-2):1-10. doi: 10.1007/s10493-007-9060-7. Epub 2007 Mar 1. Exp Appl Acarol. 2007. PMID: 17333459
-
Oribatid mite communities in mountain scree: stable isotopes (15N, 13C) reveal three trophic levels of exclusively sexual species.Exp Appl Acarol. 2021 Mar;83(3):375-386. doi: 10.1007/s10493-021-00597-4. Epub 2021 Mar 1. Exp Appl Acarol. 2021. PMID: 33646483 Free PMC article.
-
Fifty shades of bacterial endosymbionts and some of them still remain a mystery: Wolbachia and Cardinium in oribatid mites (Acari: Oribatida).J Invertebr Pathol. 2022 Mar;189:107733. doi: 10.1016/j.jip.2022.107733. Epub 2022 Feb 17. J Invertebr Pathol. 2022. PMID: 35183553 Review.
-
Behavioural studies on eriophyoid mites: an overview.Exp Appl Acarol. 2010 Jul;51(1-3):31-59. doi: 10.1007/s10493-009-9319-2. Epub 2009 Sep 25. Exp Appl Acarol. 2010. PMID: 19779863 Review.
Cited by
-
Mites Living in the Nests of the White Stork and Black Stork in Microhabitats of the Forest Environment and Agrocenoses.Animals (Basel). 2023 Oct 12;13(20):3189. doi: 10.3390/ani13203189. Animals (Basel). 2023. PMID: 37893913 Free PMC article.
-
Parthenogenetic vs. sexual reproduction in oribatid mite communities.Ecol Evol. 2019 May 29;9(12):7324-7332. doi: 10.1002/ece3.5303. eCollection 2019 Jun. Ecol Evol. 2019. PMID: 31380053 Free PMC article.
-
Positive correlation of trophic level and proportion of sexual taxa of oribatid mites (Acari: Oribatida) in alpine soil systems.Exp Appl Acarol. 2014 Aug;63(4):465-79. doi: 10.1007/s10493-014-9801-3. Epub 2014 Apr 1. Exp Appl Acarol. 2014. PMID: 24687174
-
Older Lineages of Oribatid Mites in Mountain Ranges Have Broader Geographic Ranges and Exhibit More Generalistic Traits.Ecol Evol. 2025 Feb 28;15(3):e71046. doi: 10.1002/ece3.71046. eCollection 2025 Mar. Ecol Evol. 2025. PMID: 40027424 Free PMC article.
-
Temporal fluctuations in oribatid mites indicate that density-independent factors favour parthenogenetic reproduction.Exp Appl Acarol. 2016 Apr;68(4):387-407. doi: 10.1007/s10493-015-0001-6. Epub 2016 Jan 6. Exp Appl Acarol. 2016. PMID: 26739694
References
-
- André HM. Notes on the ecology of corticolous epiphyte dwellers. 3. Oribatida. Acarologia. 1984;25:385–395.
-
- André HM. Associations between corticolous microarthoropod communities and epiphytic cover on bark. Holarct Ecol. 1985;8:113–119.
-
- Avise JC. Clonality. The genetics, ecology and evolution of sexual abstinence in vertebrate animals. Oxford: Oxford University Press; 2008.
-
- Beckmann M. The development of the soil mesofauna of a ruderal ecosystem as influenced by reclamation measures: 1. Oribatids—Acari: Oribatei. Pedobiologia. 1988;31:391–408.
-
- Behan-Pelletier VM, Walter DE. Biodiversity of Oribatid mites (Acari: Oribatida) in tree canopies and litter. In: Coleman DC, Hendrix PF, editors. Invertebrates as webmasters in ecosystems. Wallingfort: CABI Publishing; 2000. p. 187.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

