Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique
- PMID: 20490627
- PMCID: PMC3016610
- DOI: 10.1007/s11095-010-0169-8
Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique
Abstract
Purpose: Design and evaluation of a novel laser-based method for micromoulding of microneedle arrays from polymeric materials under ambient conditions. The aim of this study was to optimise polymeric composition and assess the performance of microneedle devices that possess different geometries.
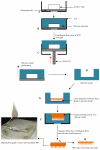
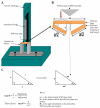
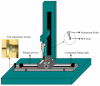
Methods: A range of microneedle geometries was engineered into silicone micromoulds, and their physicochemical features were subsequently characterised.
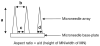
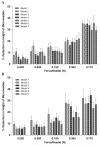
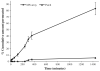
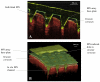
Results: Microneedles micromoulded from 20% w/w aqueous blends of the mucoadhesive copolymer Gantrez® AN-139 were surprisingly found to possess superior physical strength than those produced from commonly used pharma polymers. Gantrez® AN-139 microneedles, 600 μm and 900 μm in height, penetrated neonatal porcine skin with low application forces (>0.03 N per microneedle). When theophylline was loaded into 600 μm microneedles, 83% of the incorporated drug was delivered across neonatal porcine skin over 24 h. Optical coherence tomography (OCT) showed that drug-free 600 μm Gantrez® AN-139 microneedles punctured the stratum corneum barrier of human skin in vivo and extended approximately 460 µm into the skin. However, the entirety of the microneedle lengths was not inserted.
Conclusion: In this study, we have shown that a novel laser engineering method can be used in micromoulding of polymeric microneedle arrays. We are currently carrying out an extensive OCT-informed study investigating the influence of microneedle array geometry on skin penetration depth, with a view to enhanced transdermal drug delivery from optimised laser-engineered Gantrez® AN-139 microneedles.
Figures




References
-
- Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–5. - PubMed
-
- Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–7. - PubMed
-
- Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52:909–15. - PubMed
-
- Moon SJ, Lee SS, Lee HS, Kwon TH. Fabrication of microneedle array using LIGA and hot embossing process. Microsyst Technol Micro Nanosyst Informat Storage Proc Syst. 2005;11:311–8.
-
- Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

