Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression
- PMID: 20490631
- PMCID: PMC2886089
- DOI: 10.1007/s10911-010-9178-9
Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression
Abstract
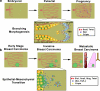
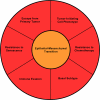
From the earliest stages of embryonic development, cells of epithelial and mesenchymal origin contribute to the structure and function of developing organs. However, these phenotypes are not always permanent, and instead, under the appropriate conditions, epithelial and mesenchymal cells convert between these two phenotypes. These processes, termed Epithelial-Mesenchymal Transition (EMT), or the reverse Mesenchymal-Epithelial Transition (MET), are required for complex body patterning and morphogenesis. In addition, epithelial plasticity and the acquisition of invasive properties without the full commitment to a mesenchymal phenotype are critical in development, particularly during branching morphogenesis in the mammary gland. Recent work in cancer has identified an analogous plasticity of cellular phenotypes whereby epithelial cancer cells acquire mesenchymal features that permit escape from the primary tumor. Because local invasion is thought to be a necessary first step in metastatic dissemination, EMT and epithelial plasticity are hypothesized to contribute to tumor progression. Similarities between developmental and oncogenic EMT have led to the identification of common contributing pathways, suggesting that the reactivation of developmental pathways in breast and other cancers contributes to tumor progression. For example, developmental EMT regulators including Snail/Slug, Twist, Six1, and Cripto, along with developmental signaling pathways including TGF-beta and Wnt/beta-catenin, are misexpressed in breast cancer and correlate with poor clinical outcomes. This review focuses on the parallels between epithelial plasticity/EMT in the mammary gland and other organs during development, and on a selection of developmental EMT regulators that are misexpressed specifically during breast cancer.
Figures




References
-
- Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120(11):1351–83. - PubMed
-
- Yamanaka Y, Ralston A, Stephenson RO, Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. 2006;235(9):2301–14. - PubMed
-
- Muller HA. Of mice, frogs and flies: generation of membrane asymmetries in early development. Dev Growth Differ. 2001;43(4):327–42. - PubMed
-
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15(6):R213–28. - PubMed
-
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233(3):706–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

