Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure
- PMID: 20490740
- PMCID: PMC2891122
- DOI: 10.1007/s12576-010-0092-0
Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure
Abstract
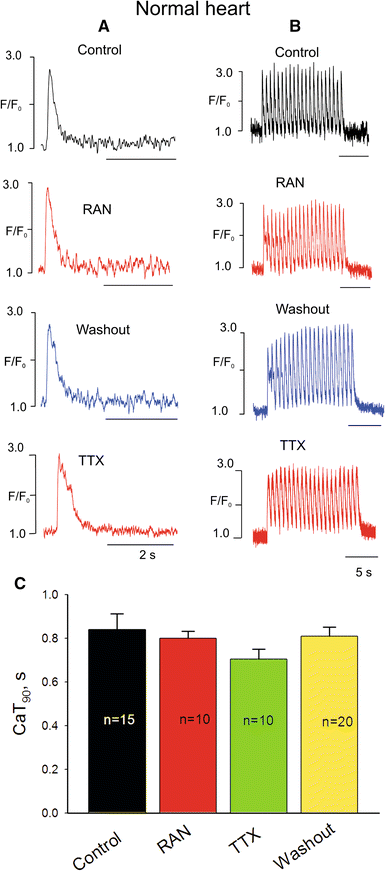
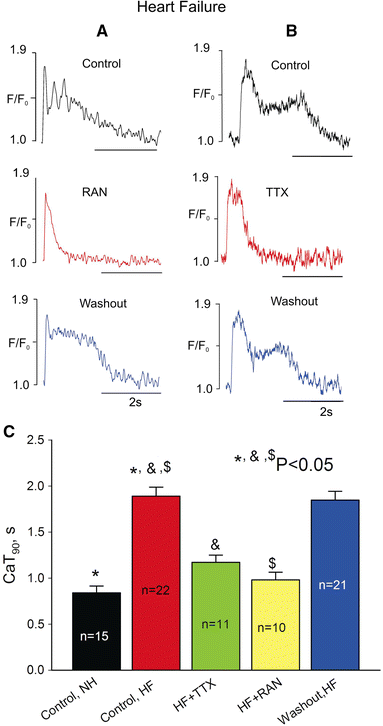
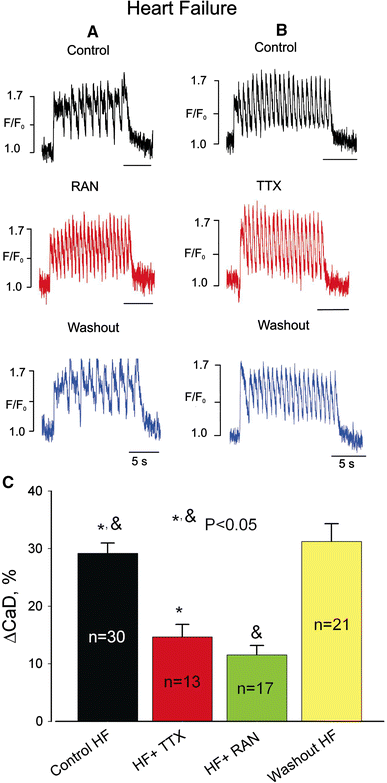
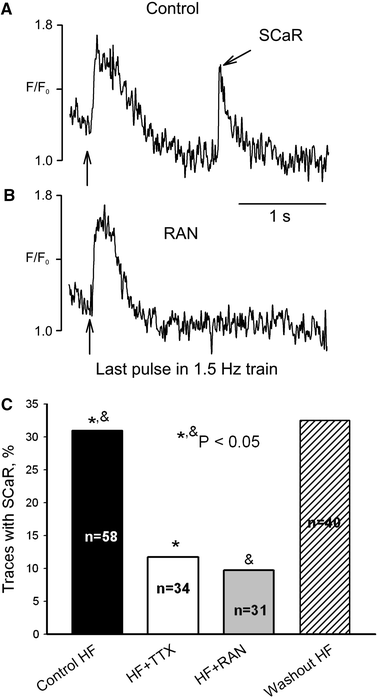
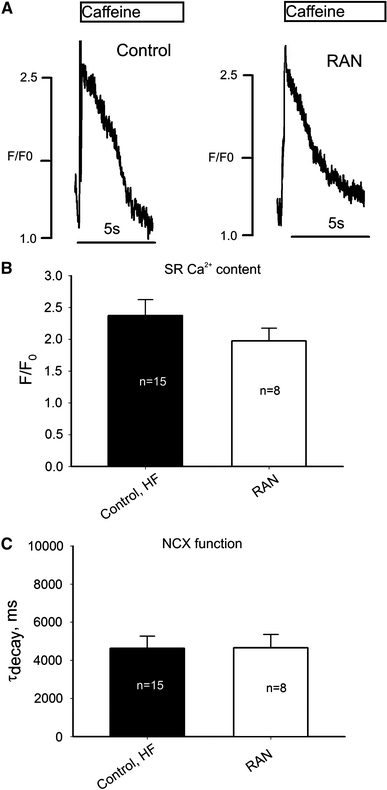
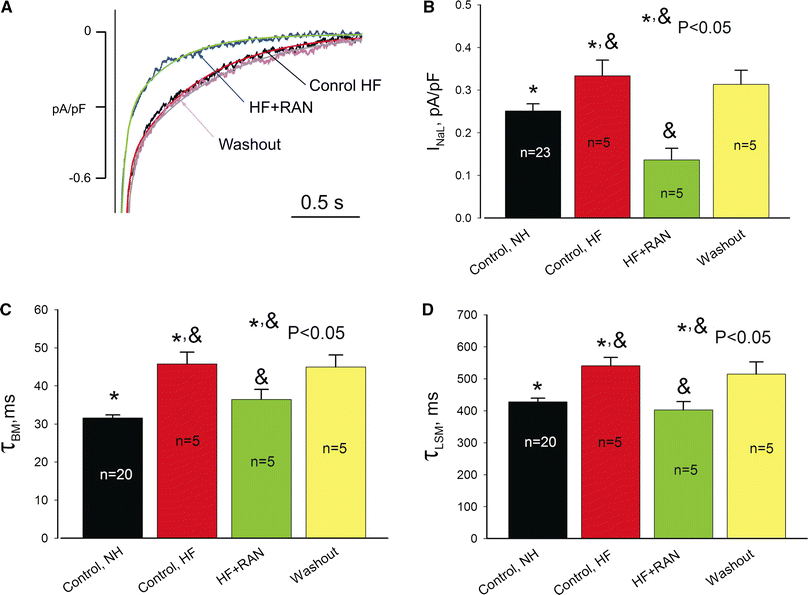
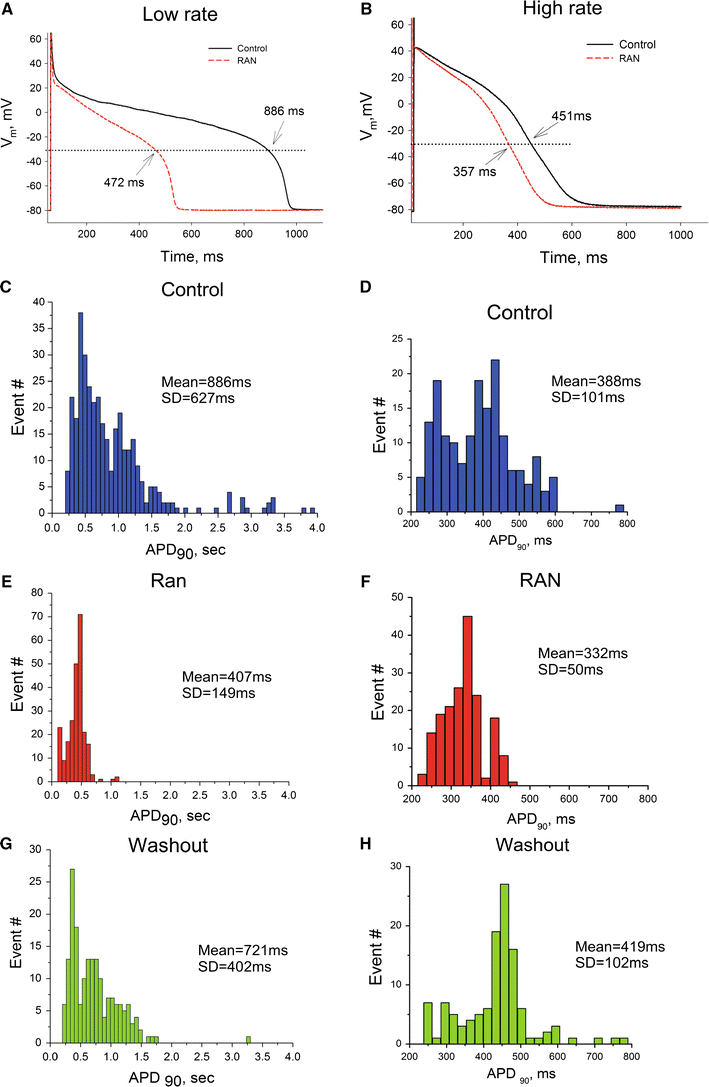
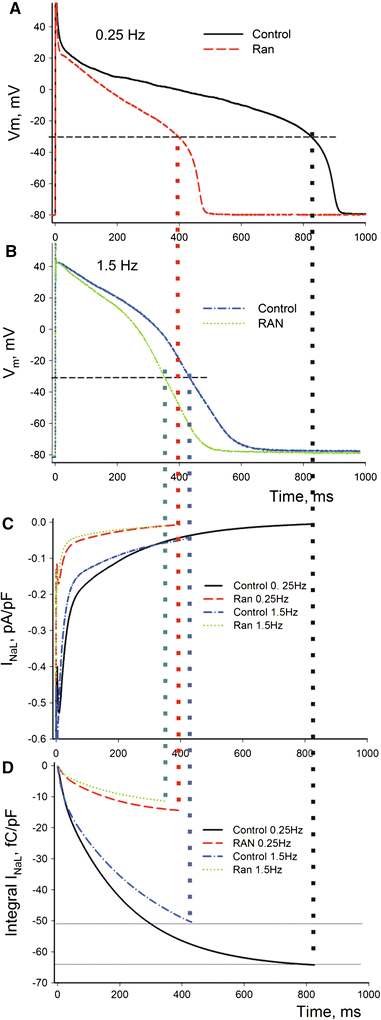
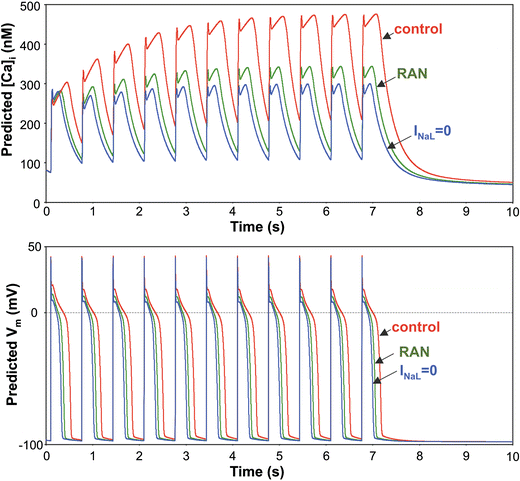
We elucidate the role of late Na+ current (INaL) for diastolic intracellular Ca2+ (DCa) accumulation in chronic heart failure (HF). HF was induced in 19 dogs by multiple coronary artery microembolizations; 6 normal dogs served as control. Ca2+ transients were recorded in field-paced (0.25 or 1.5 Hz) fluo-4-loaded ventricular myocytes (VM). INaL and action potentials were recorded by patch-clamp. Failing VM, but not normal VM, exhibited (1) prolonged action potentials and Ca2+ transients at 0.25 Hz, (2) substantial DCa accumulation at 1.5 Hz, and (3) spontaneous Ca2+ releases, which occurred after 1.5 Hz stimulation trains in ~31% cases. Selective INaL blocker ranolazine (10 microM) or the prototypical Na+ channel blocker tetrodotoxin (2 microM) reversibly improved function of failing VM. The DCa accumulation and the beneficial effect of INaL blockade were reproduced in silico using an excitation-contraction coupling model. We conclude that INaL contributes to diastolic Ca2+ accumulation and spontaneous Ca2+ release in HF.
Figures
References
-
- Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. - PubMed
-
- Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, et al. Relationship between Na+–Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

