Phylogenomic analysis of the Chlamydomonas genome unmasks proteins potentially involved in photosynthetic function and regulation
- PMID: 20490922
- PMCID: PMC2947710
- DOI: 10.1007/s11120-010-9555-7
Phylogenomic analysis of the Chlamydomonas genome unmasks proteins potentially involved in photosynthetic function and regulation
Abstract
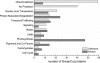
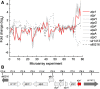
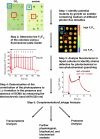
Chlamydomonas reinhardtii, a unicellular green alga, has been exploited as a reference organism for identifying proteins and activities associated with the photosynthetic apparatus and the functioning of chloroplasts. Recently, the full genome sequence of Chlamydomonas was generated and a set of gene models, representing all genes on the genome, was developed. Using these gene models, and gene models developed for the genomes of other organisms, a phylogenomic, comparative analysis was performed to identify proteins encoded on the Chlamydomonas genome which were likely involved in chloroplast functions (or specifically associated with the green algal lineage); this set of proteins has been designated the GreenCut. Further analyses of those GreenCut proteins with uncharacterized functions and the generation of mutant strains aberrant for these proteins are beginning to unmask new layers of functionality/regulation that are integrated into the workings of the photosynthetic apparatus.
Figures
Similar articles
-
Building the GreenCut2 suite of proteins to unmask photosynthetic function and regulation.Microbiology (Reading). 2019 Jul;165(7):697-718. doi: 10.1099/mic.0.000788. Epub 2019 May 7. Microbiology (Reading). 2019. PMID: 31063126 Review.
-
The Chlamydomonas genome reveals the evolution of key animal and plant functions.Science. 2007 Oct 12;318(5848):245-50. doi: 10.1126/science.1143609. Science. 2007. PMID: 17932292 Free PMC article.
-
Large-scale insertional mutagenesis of Chlamydomonas supports phylogenomic functional prediction of photosynthetic genes and analysis of classical acetate-requiring mutants.Plant J. 2015 Apr;82(2):337-51. doi: 10.1111/tpj.12806. Plant J. 2015. PMID: 25711437
-
Functional genomics of plant photosynthesis in the fast lane using Chlamydomonas reinhardtii.Trends Plant Sci. 2001 Aug;6(8):364-71. doi: 10.1016/s1360-1385(01)02018-0. Trends Plant Sci. 2001. PMID: 11495790 Review.
-
The Chlamydomonas genome project: a decade on.Trends Plant Sci. 2014 Oct;19(10):672-80. doi: 10.1016/j.tplants.2014.05.008. Epub 2014 Jun 17. Trends Plant Sci. 2014. PMID: 24950814 Free PMC article. Review.
Cited by
-
A novel proteinase, SNOWY COTYLEDON4, is required for photosynthetic acclimation to higher light intensities in Arabidopsis.Plant Physiol. 2013 Oct;163(2):732-45. doi: 10.1104/pp.113.216036. Epub 2013 Aug 12. Plant Physiol. 2013. PMID: 23940253 Free PMC article.
-
Antenna proton sensitivity determines photosynthetic light harvesting strategy.J Exp Bot. 2018 Aug 14;69(18):4483-4493. doi: 10.1093/jxb/ery240. J Exp Bot. 2018. PMID: 29955883 Free PMC article.
-
The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana.PLoS One. 2015 Apr 2;10(4):e0121658. doi: 10.1371/journal.pone.0121658. eCollection 2015. PLoS One. 2015. PMID: 25835989 Free PMC article.
-
The Arabidopsis Protein CGL20 Is Required for Plastid 50S Ribosome Biogenesis.Plant Physiol. 2020 Mar;182(3):1222-1238. doi: 10.1104/pp.19.01502. Epub 2020 Jan 14. Plant Physiol. 2020. PMID: 31937683 Free PMC article.
-
Chloroplast ATP synthase: From structure to engineering.Plant Cell. 2024 Oct 3;36(10):3974-3996. doi: 10.1093/plcell/koae081. Plant Cell. 2024. PMID: 38484126 Free PMC article. Review.
References
-
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science. 2004;306:79–86. - PubMed
-
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. - PubMed
-
- Asamizu E, Nakamura Y, Sato S, Fukuzawa H, Tabata S. A large scale structural analysis of cDNAs in a unicellular green alga Chlamydomonas reinhardtii. Generation of 3, 433 non-redundant expressed sequence tags. DNA Res. 1999;6:369–373. - PubMed
-
- Asamizu E, Miura K, Kucho K, Inoue Y, Fukuzawa H, Ohyama K, et al. Generation of expressed sequence tags from low-CO2 and high-CO2 adapted cells of Chlamydomonas reinhardtii. DNA Res. 2000;7:305–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

