Heterogeneity of the tumor vasculature
- PMID: 20490982
- PMCID: PMC3278036
- DOI: 10.1055/s-0030-1253454
Heterogeneity of the tumor vasculature
Abstract
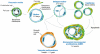
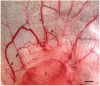
The blood vessels supplying tumors are strikingly heterogeneous and differ from their normal counterparts with respect to organization, structure, and function. Six distinctly different tumor vessel types have been identified, and much has been learned about the steps and mechanisms by which they form. Four of the six vessel types (mother vessels, capillaries, glomeruloid microvascular proliferations, and vascular malformations) develop from preexisting normal venules and capillaries by angiogenesis. The two remaining vessel types (feeder arteries and draining veins) develop from arterio-venogenesis, a parallel, poorly understood process that involves the remodeling of preexisting arteries and veins. All six of these tumor vessel types can be induced to form sequentially in normal mouse tissues by an adenoviral vector expressing vascular endothelial growth factor (VEGF)-A164. Current antiangiogenic cancer therapies directed at VEGF-A or its receptors have been of only limited benefit to cancer patients, perhaps because they target only the endothelial cells of the tumor blood vessel subset that requires exogenous VEGF-A for maintenance. A goal of future work is to identify therapeutic targets on tumor blood vessel endothelial cells that have lost this requirement.
Thieme Medical Publishers.
Figures




References
-
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. - PubMed
-
- Leenders WP, Küsters B, de Waal RM. Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium. 2002;9(2):83–87. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. - PubMed
-
- Warren B. The vascular morphology of tumors. In: Peterson H-I, editor. Tumor Blood Circulation: Angiogenesis, Vascular Morphology and Blood Flow of Experimental and Human Tumors. CRC Press; Boca Raton, FL: 1979. pp. 1–47.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

