Low thymic output in the 22q11.2 deletion syndrome measured by CCR9+CD45RA+ T cell counts and T cell receptor rearrangement excision circles
- PMID: 20491792
- PMCID: PMC2940154
- DOI: 10.1111/j.1365-2249.2010.04152.x
Low thymic output in the 22q11.2 deletion syndrome measured by CCR9+CD45RA+ T cell counts and T cell receptor rearrangement excision circles
Abstract
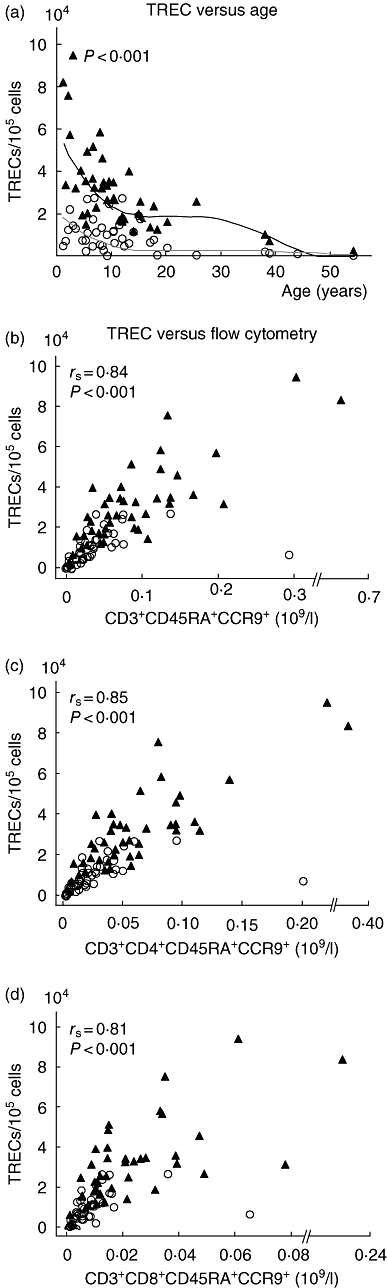
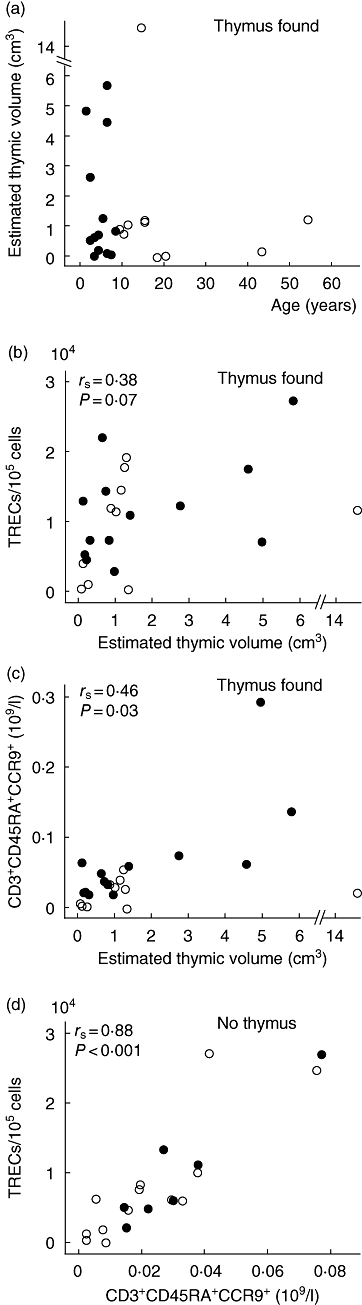
Thymic hypoplasia is a frequent feature of the 22q11.2 deletion syndrome, but we know little about patients' age-related thymic output and long-term consequences for their immune system. We measured the expression of T cell receptor rearrangement excision circles (TREC) and used flow cytometry for direct subtyping of recent thymic emigrant (RTE)-related T cells in 43 patients (aged 1-54 years; median 9 years) from all over Norway and in age-matched healthy controls. Thymic volumes were estimated by ultrasound in patients. TREC levels correlated well with RTE-related T cells defined by co-expression of CD3, CD45RA and CCR9 (r=0.84) as well as with the CD4+ and CD8+ T cell subtypes. RTE-related T cell counts also paralleled age-related TREC reductions. CD45RA+ T cells correlated well with absolute counts of CD4+ (r=0.87) and CD8+ (r=0.75) RTE-related T cells. Apart from CD45RA- T cells, all T cell subsets were lower in patients than in controls. Thymic volumes correlated better with RTE-related cells (r=0.46) than with TREC levels (r=0.38). RTE-related T cells and TREC levels also correlated well (r=0.88) in patients without an identifiable thymus. Production of RTEs is impaired in patients with a 22q11.2 deletion, and CCR9 appears to be a good marker for RTE-related T cells.
Figures

References
-
- Scambler PJ, Kelly D, Lindsay E, et al. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–9. - PubMed
-
- Halford S, Wadey R, Roberts C, et al. Isolation of a putative transcriptional regulator from the region of 22q11 deleted in DiGeorge syndrome, Shprintzen syndrome and familial congenital heart disease. Hum Mol Genet. 1993;2:2099–107. - PubMed
-
- Botto LD, May K, Fernhoff PM, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

