Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products
- PMID: 20492604
- PMCID: PMC3010325
- DOI: 10.1111/j.1537-2995.2010.02689.x
Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products
Abstract
Background: Recent evidence suggests that storage-induced alterations of the red blood cell (RBC) are associated with adverse consequences in susceptible hosts. As RBCs have been shown to form advanced glycation end products (AGEs) after increased oxidative stress and under pathologic conditions, we examined whether stored RBCs undergo modification with the specific AGE N-(carboxymethyl)lysine (N(ε) -CML) during standard blood banking conditions.
Study design and methods: Purified, fresh RBCs from volunteers were compared to stored RBCs (35-42 days old) obtained from the blood bank. N(ε) -CML formation was quantified using a competitive enzyme-linked immunosorbent assay. The receptor for advanced glycation end products (RAGE) was detected in human pulmonary microvascular endothelial cells (HMVEC-L) by real-time polymerase chain reaction, Western blotting, and flow cytometry. Intracellular reactive oxygen species (ROS) generation was measured by the use of 5-(and 6-)chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester-based assays.
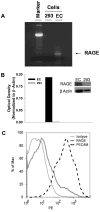
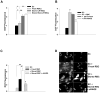
Results: Stored RBCs showed increased surface N(ε) -CML formation when compared with fresh RBCs. HMVEC-L showed detectable surface RAGE expression constitutively. When compared to fresh RBCs, stored RBCs triggered increased intracellular ROS generation in both human umbilical vein endothelial cells and HMVEC-L. RBC-induced endothelial ROS generation was attenuated in the presence of soluble RAGE or RAGE blocking antibody.
Conclusions: The formation of the AGE N(ε) -CML on the surface of stored RBCs is one functional consequence of the storage lesion. AGE-RAGE interactions may be one mechanism by which transfused RBCs cause endothelial cell damage.
© 2010 American Association of Blood Banks.
Conflict of interest statement
Figures

Similar articles
-
Differential effect of plasma or erythrocyte AGE-ligands of RAGE on expression of transcripts for receptor isoforms.Diabetes Metab. 2009 Nov;35(5):410-7. doi: 10.1016/j.diabet.2009.04.009. Epub 2009 Oct 7. Diabetes Metab. 2009. PMID: 19815443
-
The receptor for advanced glycation end products mediates lung endothelial activation by RBCs.Am J Physiol Lung Cell Mol Physiol. 2013 Feb 15;304(4):L250-63. doi: 10.1152/ajplung.00278.2012. Epub 2012 Dec 28. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23275625 Free PMC article.
-
Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor.Cardiovasc Diabetol. 2013 Aug 28;12:125. doi: 10.1186/1475-2840-12-125. Cardiovasc Diabetol. 2013. PMID: 23984879 Free PMC article.
-
The receptor for advanced glycation end-products has a central role in mediating the effects of advanced glycation end-products on the development of vascular disease in diabetes mellitus.Nephrol Dial Transplant. 1996;11 Suppl 5:13-6. doi: 10.1093/ndt/11.supp5.13. Nephrol Dial Transplant. 1996. PMID: 9044300 Review.
-
Cellular and Molecular Aspects of Blood Cell-Endothelium Interactions in Vascular Disorders.Int J Mol Sci. 2020 Jul 27;21(15):5315. doi: 10.3390/ijms21155315. Int J Mol Sci. 2020. PMID: 32727002 Free PMC article. Review.
Cited by
-
The Role of α1-Microglobulin (A1M) in Erythropoiesis and Erythrocyte Homeostasis-Therapeutic Opportunities in Hemolytic Conditions.Int J Mol Sci. 2020 Sep 30;21(19):7234. doi: 10.3390/ijms21197234. Int J Mol Sci. 2020. PMID: 33008134 Free PMC article. Review.
-
Soluble receptor for advanced glycation end products as an indicator of pulmonary vascular injury after cardiac surgery.BMC Pulm Med. 2013 Dec 16;13:76. doi: 10.1186/1471-2466-13-76. BMC Pulm Med. 2013. PMID: 24341821 Free PMC article.
-
Crosstalk between red blood cells and the immune system and its impact on atherosclerosis.Biomed Res Int. 2015;2015:616834. doi: 10.1155/2015/616834. Epub 2015 Feb 4. Biomed Res Int. 2015. PMID: 25722984 Free PMC article. Review.
-
Do We Store Packed Red Blood Cells under "Quasi-Diabetic" Conditions?Biomolecules. 2021 Jul 5;11(7):992. doi: 10.3390/biom11070992. Biomolecules. 2021. PMID: 34356616 Free PMC article. Review.
-
Molecular modifications to mitigate oxidative stress and improve red blood cell storability.Front Physiol. 2024 Oct 30;15:1499308. doi: 10.3389/fphys.2024.1499308. eCollection 2024. Front Physiol. 2024. PMID: 39539958 Free PMC article. Review.
References
-
- Dumaswala UJ, Wilson MJ, Wu YL, Wykle J, Zhuo L, Douglass LM, Daleke DL. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33(5):517–29. - PubMed
-
- Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–27. - PubMed
-
- Dumaswala UJ, Zhuo L, Jacobsen DW, Jain SK, Sukalski KA. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radic Biol Med. 1999;27(9–10):1041–9. - PubMed
-
- Dumaswala UJ, Zhuo L, Mahajan S, Nair PN, Shertzer HG, Dibello P, Jacobsen DW. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol. 2001;280(4):C867–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

