Methylmercury induced toxicogenomic response in C57 and SWV mouse embryos undergoing neural tube closure
- PMID: 20493249
- PMCID: PMC2924281
- DOI: 10.1016/j.reprotox.2010.05.009
Methylmercury induced toxicogenomic response in C57 and SWV mouse embryos undergoing neural tube closure
Abstract
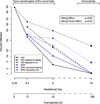
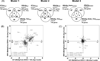
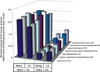
Methylmercury (MeHg) is a developmental neurotoxicant and teratogen and is hypothesized to perturb a wide range of biological processes, like other metals including arsenic (As) and cadmium (Cd). Common inbred mouse strains including C57 (sensitive) and SWV (resistant) display differences in sensitivity to metals such as As and Cd when exposed during neurulation. In this study, we investigated the impact of MeHg on neurulation, assessing for potential differences in sensitivity and associated toxicogenomic response in C57 and SWV mouse embryos. Parallel with morphological assessments of neural tube closure, we evaluated quantitative differences in MeHg-induced alterations in expression between strains at the gene level and within gene-enriched biological processes. Specifically, we observed differing sensitivities to MeHg-induced impacts on neural tube closure between C57 and SWV embryos in a time-dependent manner. These observations correlated with greater impact on the expression of genes associated with development and environmental stress-related pathways in the C57 compared to the SWV. Additional developmental parameters (e.g. mortality, growth effects) evaluated showed mixed significant effects across the two strains and did not support observations of differential sensitivity to MeHg. This study provides potential insights into MeHg-induced mechanisms of developmental toxicity, alterations associated with increased MeHg sensitivity and common biological processes affected by metals in embryos undergoing neurulation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Arsenic- and cadmium-induced toxicogenomic response in mouse embryos undergoing neurulation.Toxicol Appl Pharmacol. 2011 Jan 15;250(2):117-29. doi: 10.1016/j.taap.2010.09.018. Epub 2010 Sep 29. Toxicol Appl Pharmacol. 2011. PMID: 20883709 Free PMC article.
-
Cadmium-induced differential toxicogenomic response in resistant and sensitive mouse strains undergoing neurulation.Toxicol Sci. 2009 Jan;107(1):206-19. doi: 10.1093/toxsci/kfn221. Epub 2008 Oct 29. Toxicol Sci. 2009. PMID: 18974090 Free PMC article.
-
A systems-based approach to investigate dose- and time-dependent methylmercury-induced gene expression response in C57BL/6 mouse embryos undergoing neurulation.Birth Defects Res B Dev Reprod Toxicol. 2010 Jun;89(3):188-200. doi: 10.1002/bdrb.20241. Birth Defects Res B Dev Reprod Toxicol. 2010. PMID: 20540155 Free PMC article.
-
Mechanics of neurulation: From classical to current perspectives on the physical mechanics that shape, fold, and form the neural tube.Birth Defects Res. 2017 Jan 30;109(2):153-168. doi: 10.1002/bdra.23557. Birth Defects Res. 2017. PMID: 27620928 Free PMC article. Review.
-
Genetic backgrounds and modifier genes of NTD mouse models: An opportunity for greater understanding of the multifactorial etiology of neural tube defects.Birth Defects Res. 2017 Jan 30;109(2):140-152. doi: 10.1002/bdra.23554. Birth Defects Res. 2017. PMID: 27768235 Review.
Cited by
-
Oral exposure to methylmercury modifies the prostatic microenvironment in adult rats.Int J Exp Pathol. 2012 Oct;93(5):354-60. doi: 10.1111/j.1365-2613.2012.00825.x. Int J Exp Pathol. 2012. PMID: 22974216 Free PMC article.
-
Genome-wide Transcriptional Analysis of Oxidative Stress-related Genes and Pathways Induced by CdTe aqQDs in Mice.Nanotheranostics. 2018 Jun 19;2(3):271-279. doi: 10.7150/ntno.24590. eCollection 2018. Nanotheranostics. 2018. PMID: 29977739 Free PMC article.
-
Arsenic- and cadmium-induced toxicogenomic response in mouse embryos undergoing neurulation.Toxicol Appl Pharmacol. 2011 Jan 15;250(2):117-29. doi: 10.1016/j.taap.2010.09.018. Epub 2010 Sep 29. Toxicol Appl Pharmacol. 2011. PMID: 20883709 Free PMC article.
-
Fundamental origins of neural tube defects with a basis in genetics and nutrition.Exp Brain Res. 2025 Mar 2;243(4):79. doi: 10.1007/s00221-025-07016-9. Exp Brain Res. 2025. PMID: 40025180 Review.
-
Differential Modulatory Effects of Methylmercury (MeHg) on Ahr-regulated Genes in Extrahepatic Tissues of C57BL/6 Mice.Biol Trace Elem Res. 2024 Nov;202(11):5071-5080. doi: 10.1007/s12011-023-04050-y. Epub 2024 Jan 10. Biol Trace Elem Res. 2024. PMID: 38197905
References
-
- Anderson C, Heike K, Colquhoun D. Recommended fish intake is potentially dangerous due to high methylmercury content of certain fish. Asia Pac J Clin Nutr. 2003;(12 Suppl):S67. - PubMed
-
- Bloom N. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can. J. Fish Aquat. Sci. 1992;49:1010–1017.
-
- Grieb TM, Bowie GL, Driscoss CT, Gloss SP, Schofield CL, Porcella DB. Factors affecting mercury accumulation in fish in the upper michigan peninsula. Environmental Toxicology and Chemistry. 1990;9(7):919–930.
-
- Myers GJ, Davidson PW, Shamlaye CF. A review of methylmercury and child development. Neurotoxicology. 1998;19(2):313–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

