Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics
- PMID: 20493519
- PMCID: PMC2888034
- DOI: 10.1016/j.cell.2010.03.054
Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics
Abstract
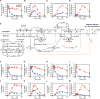
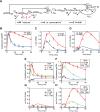
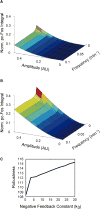
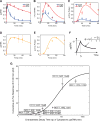
Activation of ErbB receptors by epidermal growth factor (EGF) or heregulin (HRG) determines distinct cell-fate decisions, although signals propagate through shared pathways. Using mathematical modeling and experimental approaches, we unravel how HRG and EGF generate distinct, all-or-none responses of the phosphorylated transcription factor c-Fos. In the cytosol, EGF induces transient and HRG induces sustained ERK activation. In the nucleus, however, ERK activity and c-fos mRNA expression are transient for both ligands. Knockdown of dual-specificity phosphatases extends HRG-stimulated nuclear ERK activation, but not c-fos mRNA expression, implying the existence of a HRG-induced repressor of c-fos transcription. Further experiments confirmed that this repressor is mainly induced by HRG, but not EGF, and requires new protein synthesis. We show how a spatially distributed, signaling-transcription cascade robustly discriminates between transient and sustained ERK activities at the c-Fos system level. The proposed control mechanisms are general and operate in different cell types, stimulated by various ligands.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Brondello JM, Brunet A, Pouyssegur J, McKenzie FR. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J Biol Chem. 1997;272:1368–1376. - PubMed
-
- Brondello JM, McKenzie FR, Sun H, Tonks NK, Pouyssegur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. - PubMed
-
- Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

