The leucokinin pathway and its neurons regulate meal size in Drosophila
- PMID: 20493701
- PMCID: PMC2896026
- DOI: 10.1016/j.cub.2010.04.039
The leucokinin pathway and its neurons regulate meal size in Drosophila
Abstract
Background: Total food intake is a function of meal size and meal frequency, and adjustments to these parameters allow animals to maintain a stable energy balance in changing environmental conditions. The physiological mechanisms that regulate meal size have been studied in blowflies but have not been previously examined in Drosophila.
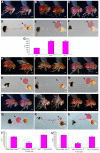
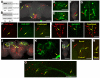
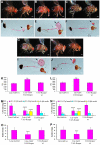
Results: Here we show that mutations in the leucokinin neuropeptide (leuc) and leucokinin receptor (lkr) genes cause phenotypes in which Drosophila adults have an increase in meal size and a compensatory reduction in meal frequency. Because mutant flies take larger but fewer meals, their caloric intake is the same as that of wild-type flies. The expression patterns of the leuc and lkr genes identify small groups of brain neurons that regulate this behavior. Leuc-containing presynaptic terminals are found close to Lkr neurons in the brain and ventral ganglia, suggesting that they deliver Leuc peptide to these neurons. Lkr neurons innervate the foregut. Flies in which Leuc or Lkr neurons are ablated have defects identical to those of leucokinin pathway mutants.
Conclusions: Our data suggest that the increase in meal size in leuc and lkr mutants is due to a meal termination defect, perhaps arising from impaired communication of gut distension signals to the brain. Leucokinin and the leucokinin receptor are homologous to vertebrate tachykinin and its receptor, and injection of tachykinins reduces food consumption. Our results suggest that the roles of the tachykinin system in regulating food intake might be evolutionarily conserved between insects and vertebrates.
2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Murphy K, Bloom S. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444(7121):854–9. - PubMed
-
- Phillips R, Powley T. Gastric volume rather than nutrient content inhibits food intake. Am. J. Physiol. 1996;271:R766–779. - PubMed
-
- Smith G. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. - PubMed
-
- Smith G. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

