Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p
- PMID: 20493815
- PMCID: PMC2877039
- DOI: 10.1016/j.devcel.2010.03.016
Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p
Abstract
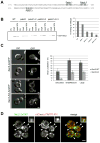
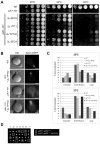
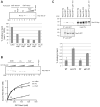
Sec2p is the guanine nucleotide exchange factor (GEF) that activates the Rab GTPase Sec4p on secretory vesicles. Sec2p also binds a Rab acting earlier in the secretory pathway, Ypt32-GTP, forming a Rab GEF cascade. Ypt32p and the Sec4p effector Sec15p (a component of the exocyst complex) compete for binding to Sec2p. Indeed Ypt32p initially recruits Sec2p, but subsequently allows a handoff of active Sec2p/Sec4p to Sec15p. Intriguingly, Golgi-associated phosphatidylinositol 4-phosphate (PI4P) works together with Ypt32-GTP in this context. PI4P inhibits Sec2p-Sec15p interactions, promoting recruitment of Sec2p by Ypt32p as secretory vesicles form. However, PI4P levels appear to decline as vesicles reach secretory sites, allowing Sec15p to replace Ypt32p as vesicles mature. In this way, the regulation of PI4P levels may switch Sec2p/Sec4p function during vesicle maturation, from a Rab GEF recruitment cascade involving Ypt32p to an effector positive feedback loop involving Sec15p.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):19995-20002. doi: 10.1073/pnas.1320029110. Epub 2013 Nov 18. Proc Natl Acad Sci U S A. 2013. PMID: 24248333 Free PMC article.
-
The casein kinases Yck1p and Yck2p act in the secretory pathway, in part, by regulating the Rab exchange factor Sec2p.Mol Biol Cell. 2016 Feb 15;27(4):686-701. doi: 10.1091/mbc.E15-09-0651. Epub 2015 Dec 23. Mol Biol Cell. 2016. PMID: 26700316 Free PMC article.
-
Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast.J Cell Biol. 2002 Jun 10;157(6):1005-15. doi: 10.1083/jcb.200201003. Epub 2002 Jun 3. J Cell Biol. 2002. PMID: 12045183 Free PMC article.
-
Regulation of membrane traffic by Rab GEF and GAP cascades.Small GTPases. 2016 Oct;7(4):252-256. doi: 10.1080/21541248.2016.1213781. Epub 2016 Jul 18. Small GTPases. 2016. PMID: 27427966 Free PMC article. Review.
-
Who's in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond.J Cell Biol. 2021 Sep 6;220(9):e202105120. doi: 10.1083/jcb.202105120. Epub 2021 Aug 12. J Cell Biol. 2021. PMID: 34383013 Free PMC article. Review.
Cited by
-
βIII spectrin regulates the structural integrity and the secretory protein transport of the Golgi complex.J Biol Chem. 2013 Jan 25;288(4):2157-66. doi: 10.1074/jbc.M112.406462. Epub 2012 Dec 11. J Biol Chem. 2013. PMID: 23233669 Free PMC article.
-
Exploring the consequences of redirecting an exocytic Rab onto endocytic vesicles.Mol Biol Cell. 2023 May 1;34(5):ar38. doi: 10.1091/mbc.E23-01-0037. Epub 2023 Mar 1. Mol Biol Cell. 2023. PMID: 36857153 Free PMC article.
-
Signaling at the Golgi.Cold Spring Harb Perspect Biol. 2011 May 1;3(5):a005314. doi: 10.1101/cshperspect.a005314. Cold Spring Harb Perspect Biol. 2011. PMID: 21454247 Free PMC article. Review.
-
Suppression of Vps13 adaptor protein mutants reveals a central role for PI4P in regulating prospore membrane extension.PLoS Genet. 2021 Aug 18;17(8):e1009727. doi: 10.1371/journal.pgen.1009727. eCollection 2021 Aug. PLoS Genet. 2021. PMID: 34407079 Free PMC article.
-
Requirement of Phosphoinositides Containing Stearic Acid To Control Cell Polarity.Mol Cell Biol. 2016 Feb 16;36(5):765-80. doi: 10.1128/MCB.00843-15. Epub 2015 Dec 28. Mol Cell Biol. 2016. PMID: 26711260 Free PMC article.
References
-
- Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Developmental cell. 2002;2:593–605. - PubMed
-
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999a;397:621–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

